
Effects of environmental factors and omega-3 fatty acids on rheumatoid arthritis
Introduction
With a prevalence that has been estimated to be 0.5%±0.2% and a female predominance, rheumatoid arthritis (RA) represents the most common chronic systemic autoimmune rheumatism. Typically, RA is characterized by polyarticular pain, morning stiffness, fatigue, joint and bone inflammation and destruction (1). A more severe evolution is encountered in RA patients with an age lower than 50 years at diagnosis, anti-cyclic citrullinated peptides (anti-CCP) autoantibody (Ab) positivity, IgM rheumatoid factor (RF) at elevated levels (>50 UI/mL), and bone erosions (2,3). RA appears to present geographical variations with an apparent reduction from north to the south, and from urban to rural areas (4).
The deregulated immunological mechanisms leading to RA are complex and include primarily an abnormal cross-talk between antigen-presenting cells (APCs), T cells, and B cells which in turn contributes to the expansion of autoreactive lymphocytes, and to the production of high amounts of pro-inflammatory cytokines (Figure 1). After several months or years (5), persistent inflammatory arthritis leads to synovial hyperplasia, cartilage destruction, bone erosion, and neutrophil recruitment. In those patients with anti-CCP Ab and RF, referred to as Ab seropositivity (60–80%), such association is associated with more severe symptoms and joint damage, indeed the formation of immune complexes containing citrullinated peptides/anti-CCP Ab/RF can lead to abundant complement activation.
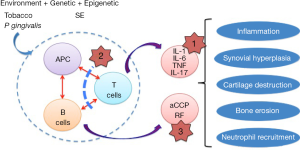
Moreover, there is consistent information from the literature linking RA development with accelerating factors such as smoking, alcohol consumption, obesity, exposure to air pollution, a low level of formal education, and infections (6,7). At the opposite end, long-chain omega-3 polyunsaturated fatty acids [omega-3 fatty acids (OA3FA)] have a protective effect for RA development (8), and it has been further reported that OA3FA protects children at risk for type 1 diabetes from the development of pre-clinical islet autoimmunity (9).
Control of the immune system in RA by genetic and epigenetic factors
The use of monozygotic (MZ) twins and the determination of the concordance rate (CR), which tests the proportion of affected pairs in a disease, highlight the importance of the environmental component in RA (CR 10–40%). In contrast, an elevated CR characterizes autoimmune diseases with higher genetic components such as celiac disease (CR 75–85%) and psoriasis (CR 40–65%) (10).
The development of serological tools in the 1970s and the molecular biology tools in the 1980s have permitted the revelation of associations between RA and the shared epitope (SE) driven by the human leukocyte antigen (HLA) antigen D related region (DR) B1 (11). Several arguments support the possibility that citrullinated peptides interact with the peptide-binding groove of the SE, trigger autoreactive T-cell clonal expansion, and contribute to the production of specific anti-CCP Ab. The association between SE and both anti-CCP Ab and RF is observed in 2/3 of patients with RA and an elegant HLA-DRB1 transgenic mouse model recapitulates the disease in the presence of citrullinated peptides (12). The association between anti-CCP Ab and RF negative RA patients and SE is poor. Furthermore, citrullinated peptide presentation of the SE can be influenced by common oral bacteria present within the dental plaque such as Porphyromonas gingivalis that promotes aberrant levels of citrullinated peptides by producing peptidylarginine deiminase enzymes which promotes arginine to citrulline conversion and then a local breach of tolerance, process amplified in smokers as tobacco promotes citrullinated peptide interaction with the SE (13). An elevated level of anti-CCP Ab is observed in SE-positive smokers.
Moreover, with the development of the genome-wide association study (GWAS) project, up to 100 genetic variations (SNPs) are associated with RA (14). From these genetic studies important pathways were highlighted to be crucial in the development of RA, including (i) antigen presentation (CMH/HLA); (ii) the citrullination process (e.g., PADI4); (iii) immune complex recognition and elimination (e.g., FCGR2A, FCRL3); (iv) cytokines (e.g., IL-2, IL-6R, IL-21, STAT4); (v) co-stimulatory pathways (e.g., CD28, CTLA4, CD40); and (vi) T and B cell activation (e.g., PTPN22, BLK). However, with the exception of the SE HLA-DRB1 alleles genes that have a significant odds ratio (OR =2 to 5), non-HLA gene associations are usually not disease specific, geographically restricted, and with modest OR (1.1 to 1.8) suggesting, again, a modest contribution for genetic factors in RA. Last but not least, cross-talk exists between genetic and epigenetic factors as the distribution of the RA-associated genetic risk-factors overlap in effector memory CD4+ T cells with expression quantitative trait loci (eQTL, 50%) and/or long range epigenetic-regulatory sequences (80%) present outside promoter and gene coding areas (15-17). Epigenetic modifications include DNA methylation and histone modifications, and they are defined as changes affecting gene expression that do not involve change in the DNA sequence. Epigenetic mechanisms are intrinsically reversible, highly dynamic, and cell specific (18). Although incompletely understood, mechanisms affecting epigenetic pathways in RA are cell specific, controlled in part by pro-inflammatory cytokines, and reversible following treatment (19-21).
Omega-3 fatty acids (OA3FA)
Growing evidence suggests that particular diets containing fatty fish (e.g., salmon, lake trout, sardines and tuna) are beneficial for primary and secondary prevention of RA (22). Based on these observations, dietary regimens originating from fish oils and containing the OA3FA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are available and assist in RA prevention and reduce the need for non-steroidal anti-inflammatory drugs (NSAIDs) (23). In support of these observations, the evaluation of OA3FA concentrations in tissues, especially in erythrocyte membranes, further supports that RA development is associated with lower levels of OA3FA (24), and this reduction was significantly increased when considering the anti-CCP Ab positive population at risk for future RA (25).
At least three mechanisms are described for OA3FA in RA:
- In the first mechanism, OA3FA are suspected of preventing primarily and secondarily RA through their capacity to inhibit the production of pro-inflammatory eicosanoids such as prostaglandin E2 (PGE2) and leucotriene B4 (LTB4), which in turn inhibit the activation of the nuclear factor kappa B (NF-κB), and thus the production and release of the pro-inflammatory interleukins (IL) [e.g., IL-1, IL-6, tumour necrosis factor (TNF)-alpha] that could ultimately result in the activation and maturation of autoreactive B cells and synoviocytes. OA3FA also prevents pro-inflammatory cytokine expression through the epigenetic pathway by restoring DNA methylation as demonstrated for the promoter of IL-6 (26);
- In the second mechanism, in vitro experiments support an effect of OA3FA on cell surface receptors either directly by promoting their expression [e.g., vascular cell adhesion molecule (VCAM)-1 an adhesion molecule, and PPARγ in monocytes] or repression (e.g., CCL5, HLA-DQ/DR), or indirectly through their action on the lipid rafts (e.g., IL-6 receptor, and CD28). Thereby, OA3FA reduce Th17 differentiation, enhance Foxp3+ CD4+ T cells regulatory functions, promote M2 polarization, and lower the number of NK cells (27);
- In the third mechanism, an interaction between OA3FA and the SE is also suspected and this was recently demonstrated in an epidemiological study conducted in participants at risk for RA by Gan and colleagues (8). Indeed, an inverse association between OA3FA concentrations and SE + RF (OR =0.26; 95% CI: 0.09–0.77, P=0.02), or SE + anti-CCP Ab (OR =0.44; 95% CI: 0.21–0.93, P=0.03) was reported in two cohorts at risk for RA: one selected from first relatives of probands with RA patients and another one with children possessing type 1 diabetes risk alleles, which includes SE. Furthermore, authors reported that increasing dietary OA3FA intake was protective for RF positivity in SE-positive participants (P=0.02). In contrast, no association was observed in participants who were SE-negative, or cases both positive for anti-CCP Ab and RF. The exact mechanism of this specificity and suspected cross-talk between OA3FA and SE is not completely understood and needs further exploration.
In conclusion, and according to the recently reported study by Gan and colleagues, the protective effects of OA3FA against the development of RA seems to be restricted to a subgroup of patients. As a consequence, such observations provide novel arguments for the utilization of fish oil supplementation as an adjunct therapy in selected patients with RA. However, better elucidation of the interplay between OA3FA, genetic and epigenetic factors is now required to characterize and select these patients.
Acknowledgments
The authors thank Simone Forest and Geneviève Michel for their help with the writing of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Executive Editor-in-Chief, Dongquan Shi, MD, PhD (Department of Sports Medicine and Adult Reconstruction, Drum Tower Hospital, Medical School, Nanjing University, Nanjing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2016.06.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kourilovitch M, Galarza-Maldonado C, Ortiz-Prado E. Diagnosis and classification of rheumatoid arthritis. J Autoimmun 2014;48-49:26-30. [Crossref] [PubMed]
- Renaudineau Y, Jamin C, Saraux A, et al. Rheumatoid factor on a daily basis. Autoimmunity 2005;38:11-6. [Crossref] [PubMed]
- Nielsen SF, Bojesen SE, Schnohr P, et al. Elevated rheumatoid factor and long term risk of rheumatoid arthritis: a prospective cohort study. BMJ 2012;345:e5244 [Crossref] [PubMed]
- Shapira Y, Agmon-Levin N, Shoenfeld Y. Geoepidemiology of autoimmune rheumatic diseases. Nat Rev Rheumatol 2010;6:468-76. [Crossref] [PubMed]
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380-6. [Crossref] [PubMed]
- Mikuls TR, Payne JB, Deane KD, et al. Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: The spark that lights the fire in rheumatoid arthritis? J Allergy Clin Immunol 2016;137:28-34. [Crossref] [PubMed]
- Arleevskaya MI, Gabdoulkhakova AG, Filina YV, et al. A transient peak of infections during onset of rheumatoid arthritis: a 10-year prospective cohort study. BMJ Open 2014;4:e005254 [Crossref] [PubMed]
- Gan RW, Demoruelle MK, Deane KD, et al. Omega-3 fatty acids are associated with a lower prevalence of autoantibodies in shared epitope-positive subjects at risk for rheumatoid arthritis. Ann Rheum Dis 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Norris JM, Yin X, Lamb MM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007;298:1420-8. [Crossref] [PubMed]
- Brooks WH, Le Dantec C, Pers JO, et al. Epigenetics and autoimmunity. J Autoimmun 2010;34:J207-19. [Crossref] [PubMed]
- Konsta OD, Le Dantec C, Brooks WH, et al. Genetics and Epigenetics of Autoimmune Diseases. eLS 2015:1-9.
- Hill JA, Bell DA, Brintnell W, et al. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med 2008;205:967-79. [Crossref] [PubMed]
- Kharlamova N, Jiang X, Sherina N, et al. Antibodies to Porphyromonas gingivalis Indicate Interaction Between Oral Infection, Smoking, and Risk Genes in Rheumatoid Arthritis Etiology. Arthritis Rheumatol 2016;68:604-13. [Crossref] [PubMed]
- Yamamoto K, Okada Y, Suzuki A, et al. Genetics of rheumatoid arthritis in Asia--present and future. Nat Rev Rheumatol 2015;11:375-9. [Crossref] [PubMed]
- Farh KK, Marson A, Zhu J, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 2015;518:337-43. [Crossref] [PubMed]
- Dubois PC, Trynka G, Franke L, et al. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet 2010;42:295-302. [Crossref] [PubMed]
- Konsta OD, Le Dantec C, Charras A, et al. An in silico Approach Reveals Associations between Genetic and Epigenetic Factors within Regulatory Elements in B Cells from Primary Sjögren's Syndrome Patients. Front Immunol 2015;6:437. [Crossref] [PubMed]
- Le Dantec C, Gazeau P, Mukherjee S, et al. How the environment influences epigenetics, DNA methylation, and autoimmune diseases. In: Lu Q, Chang CC, Richardson BC, editors. Epigenetics and Dermatology. Elsevier; 2015:467-85.
- Glossop JR, Emes RD, Nixon NB, et al. Genome-wide profiling in treatment-naive early rheumatoid arthritis reveals DNA methylome changes in T and B lymphocytes. Epigenomics 2016;8:209-24. [Crossref] [PubMed]
- Angiolilli C, Grabiec AM, Ferguson BS, et al. Inflammatory cytokines epigenetically regulate rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing HDAC5 expression. Ann Rheum Dis 2016;75:430-8. [Crossref] [PubMed]
- de Andres MC, Perez-Pampin E, Calaza M, et al. Assessment of global DNA methylation in peripheral blood cell subpopulations of early rheumatoid arthritis before and after methotrexate. Arthritis Res Ther 2015;17:233. [Crossref] [PubMed]
- Di Giuseppe D, Crippa A, Orsini N, et al. Fish consumption and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther 2014;16:446. [Crossref] [PubMed]
- Lee YH, Bae SC, Song GG. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch Med Res 2012;43:356-62. [Crossref] [PubMed]
- Lee AL, Park Y. The association between n-3 polyunsaturated fatty acid levels in erythrocytes and the risk of rheumatoid arthritis in Korean women. Ann Nutr Metab 2013;63:88-95. [Crossref] [PubMed]
- Gan RW, Young KA, Zerbe GO, et al. Lower omega-3 fatty acids are associated with the presence of anti-cyclic citrullinated peptide autoantibodies in a population at risk for future rheumatoid arthritis: a nested case-control study. Rheumatology (Oxford) 2016;55:367-76. [Crossref] [PubMed]
- Ma Y, Smith CE, Lai CQ, et al. The effects of omega-3 polyunsaturated fatty acids and genetic variants on methylation levels of the interleukin-6 gene promoter. Mol Nutr Food Res 2016;60:410-9. [Crossref] [PubMed]
- Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015;1851:469-84.
Cite this article as: Lemerle J, Arleevskaya MI, Brooks WH, Renaudineau Y. Effects of environmental factors and omega-3 fatty acids on rheumatoid arthritis. Ann Joint 2016;1:8.


