
Brachial plexus injuries in the contact athlete: a narrative review
Introduction
Background
Brachial plexus injuries (BPIs) are common injuries in contact athletes that represent a spectrum of damage with varying presentation, mechanism, and treatment. More devastating BPIs are seen with motorcycle and motor vehicle accidents, however, less severe neuropraxic injuries, such as “burners” or “stingers”, may occur in up to 65% of football players and 30–40% of rugby players (1-4). Clinicians who care for contact athletes should be aware of this spectrum of injuries and how to evaluate and treat them.
Injuries may occur anywhere along the brachial plexus, from the cervical nerve root to the terminal branches. In contact sports, there are three suggested primary mechanisms of injury to the brachial plexus. These include traction, direct compression of the plexus at the supraclavicular region, and cervical nerve root compression due to hyperextension or hyperflexion of the neck (3,5-10). These are well recognized across multiple sports with football and rugby amongst the most common, due to tackling (11). Contact athletes are at specific risk for this type of injury due to the vulnerable nature of hyperflexion and extension of the neck and use of the shoulder as a fulcrum of force against other athletes.
Rationale and knowledge gap
A severe BPI with permanent axon damage or ischemia is far less common than transient neuropraxic injuries, widely known as “stingers” or “burners” (12). These injuries are characterized by unilateral transient motor or sensory loss with associated burning radiating pain (8,10,13). While there is significant literature to support the commonality of the stinger injury, there is far less data to describe the care of the athlete with an axonotmesis or neurotmesis injury, including the severe root avulsion injury (6-8,14). Nonetheless, these injuries have been classified to assist with prognostication and treatment options.
Objective
Differentiating between axonotmesis and neurotmesis can present a diagnostic challenge based on clinical presentation alone (5,10). This review aims to summarize the available literature on BPIs in contact athletes from neuropraxia to neurotmesis to supply clinicians with a diagnostic algorithm, treatment considerations, and information for outcome counseling. We present this article in accordance with the Narrative Review reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-24-67/rc).
Methods
This review was performed by searching for literature available in English from inception to October 2024 within PubMed, Cochrane Review, and Google Scholar. Keywords including “contact athlete”, “contact sports”, “brachial plexus injuries”, “brachial plexus”, “sports”, “athlete”, “neuropraxia”, and “axonotmesis” were used with Boolean properties to generate a list of over 500 published manuscripts. Article titles and abstracts were reviewed by three independent authors, and many were excluded for lack of peer-reviewed status, availability only in non-English language, lack of full-text availability, or subject matter primarily focused on regional anesthesia of the brachial plexus, terminal branch peripheral nerve injuries, and thoracic outlet syndrome of the contact athlete. Meta-analyses, cohort studies, epidemiology studies, case series, case reports, and review articles were included based on their novelty in contribution to the literature, published, peer-reviewed status. Burner injuries, being far more common, were much more likely to be evaluated in the setting of larger cohort or epidemiology studies and meta-analysis. Less common axonotmesis or neurotmesis injuries were available for review in the setting of case series or reports. The research strategies used in this narrative review are summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 10/9/2024 |
| Databases and other sources searched | PubMed, Cochrane Review, and Google Scholar |
| Search terms used | “Contact athlete”, “contact sports”, “brachial plexus injuries”, “brachial plexus”, “sports”, “athlete”, “neuropraxia”, and “axonotmesis” |
| Timeframe | Inception to October 2024 |
| Inclusion criteria | Peer-reviewed, available in English |
| Selection process | Selection consensus was made by three co-authors |
| Additional considerations | Individual manuscripts references were reviewed for additional sources |
Epidemiology
Transient neuropraxias (burners/stingers) in contact sports are common, with prevalence in cross-sectional studies being estimated between 49% and 65% of football players experiencing at least one episode throughout their career (3,4,8,15). In an epidemiological study of National Football League (NFL) players from 2015 to 2019, the average single-season risk was approximately 3.74%, and another cross-sectional NFL combine study noted burners made up 4.4% of current or past injuries (7,16). This same study estimated an incidence of 11.48/100,000 player-plays. Twenty-two percent of the athletes with stingers during this period had experienced a stinger prior to this, with 12.8% having experienced two or more in the past (7). Beyond football and rugby, wrestling and ice hockey are the next most common sport to pose risk for a BPI with occasional case reports within the literature. From 1972 to 1976, 14 (2.1%) of 666 injuries due to wrestling were found to be a “pinched-nerve” syndrome of the neck (17).
However, it is still suggested that these injuries may be underreported due to fear of athletes losing playing time. While some players do miss time for injuries that do not resolve immediately, 76% of players missed no time at all (7). Additionally, athletes with burners and stingers within the study of NFL combine had an average draft position of 117, compared to an average position of 115 across the entire cohort of athletes demonstrating no significant difference (16).
Of athletes sustaining axonotmesis and neurotmesis injuries, approximately 50% had previously sustained a burner (6). This risk of recurrence is unknown, as is the risk of progression of a future event from neuropraxia to axonotmesis. Nissen et al. discussed a patient who underwent two burner events 1 week apart with an increased length of symptoms after the second event. The initial burner symptoms resolved almost immediately. However, following the second event the next week, the patient sustained symptoms up to 2 months prior to full resolution (10). Additionally, small sample size studies have described a “chronic burner syndrome” phenomenon, which may represent an unknown risk of a “second-hit” type injury, such as in the instance of concussions, where repetitive injury may lead to increased severity or prolonged duration of symptoms (3,18,19). Recurrent burner injuries have been shown to be associated with cervical foraminal stenosis or canal stenosis as explained by Reilly and Torg, however there have been no prospective or retrospective studies to demonstrate a correlation between repetitive burner events and increased risk of progression to more severe axonal injury (19-21). These authors hypothesize that there is a potential relationship between frequency of injuries and the relative severity of subsequent injuries. The relatively high incidence of burner injuries and low incidence of axonal injury may reflect a lack of causation or impact, however it warrants more investigation to assess the risk to the axonal integrity with recurrent burner injuries.
Anatomy
The brachial plexus is a complex network and confluence of spinal nerves as they exit the spinal cord. Importantly, each motor axon travels within the anterior corticospinal tract and exits via a ventral rootlet to join with the dorsal rootlet of the sensory tracts beyond the dorsal root ganglion. Within this ganglion lives the sensory cell body, a pseudounipolar neuron of which the axon of the peripheral sensory nerve and the ascending axon to the sensory cortex originate (5,22). BPIs proximal to the ganglion are known as pre-ganglionic injuries, and BPIs distal are known as post-ganglionic injuries. Pre-ganglionic injuries have poor prognosis and often involve a full avulsion of the rootlets with little to no potential for spontaneous regeneration (5,14,22,23). These injuries separate the motor axon from the cell body of the primary motor cortex at the level of the ventral root, thus resulting in motor axonal degeneration. However, the sensory peripheral axon is still in continuity with the cell body and therefore does not degenerate. Thus, it may produce signal on electromyography (EMG) even if the ascending axon to the sensory cortex has been disrupted and no sensation may be recognized by the brain (2,5,22,24).
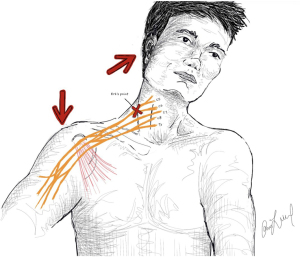
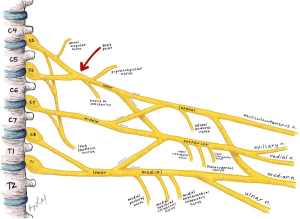
Moving distally past the ganglion, the next anatomic site of injury is the convergence of the C5 and C6 nerve roots to form the upper trunk, also known as Erb’s point (21). This point is a landmark approximately 2–3 cm superior to the clavicle at the posterior border of the sternocleidomastoid. Erb’s point is just distal to the fascial attachments of the paraspinal musculature, making it the first point of the brachial plexus to be tethered proximally and free for traction distally as seen in Figure 1 (5). Traction in this region may lead to axonotmesis or neurotmesis, which is referred to as a rupture in the post-ganglionic nerve (14). Additionally, as the anatomic location at the neck-shoulder junction, it is also a high-risk area of direction compression during tackling (7). Notably, this point is distal to the takeoff of the dorsal scapular and phrenic branches of the C5 root along with the long thoracic nerve contributed to by C5, C6, and C7 roots as depicted in Figure 2. As the most proximal post-ganglionic locale for injury, investigation of rhomboids, levator scapulae (dorsal scapular nerve), serratus anterior (long thoracic nerve), and hemidiaphragm (phrenic nerve) can aid in localization of a lesion (5,21).
Injuries to the lower trunk, comprised of the C8 and T1 nerve roots, are far less common than injuries affecting the upper trunk or even the entirety of the plexus (1,14). This is likely due to the relative abducted position of the arm and amount of energy required to place these nerve roots or trunk on stretch. They are also anatomically infra-clavicular, deeper than the upper trunk, and thus less susceptible to direct compression. There have been reports of injuries to this region of the plexus via shoulder dislocations, however these are more common with higher energy mechanisms and less common in the contact athlete (25).
The prognosis of the BPI is also affected by the damaged histologic layer of the nerve. Clancy et al. in 1977 applied the pathomechanism of injury to peripheral nerves to the brachial plexus adapting previous studies by Denny-Brown and Doherty of 1945. In these in vitro studies, peripheral nerves such as the peroneal nerve were stretched up to 100% of their original length, with injury to the endoneurium occurring just beyond this, progressing to perineurial and finally epineurial injury (8,26). This disruption of the epineurium results in damage to the vasculature of the nerve causing hematoma and ischemia (10).
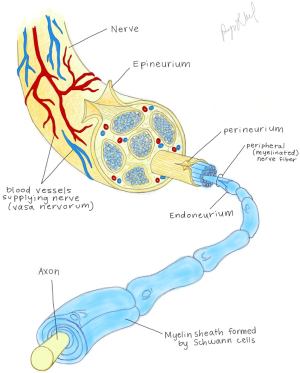
Both Seddon and Sunderland described these injuries as they relate to the layer of the peripheral nerve affected by the injury as demonstrated in Table 2 with illustrations of these nerve layers in Figure 3 (28). Neuropraxia (Seddon) and first degree (Sunderland) describe an injury to the myelin sheath. These injuries are transient and represent the burners and stingers within the current sports literature. Axonotmesis (Seddon) includes any injury of the axon up to the level of the epineurium which typically remains intact. Second, third, and fourth degree (Sunderland) include injury to the axon, along with the endoneurium and perineurium, respectively. Finally, neurotmesis (Seddon) or fifth degree injuries (Sunderland) represent injury to all parts of the peripheral nerve from the axon through the endoneurium, perineurium, and epineurium (3,10,27). Increased degree of severity or elevation from axonotmesis to neurotmesis worsens prognosis for spontaneous recovery as it reflects more disruption to the connective tissues and vasculature that protect organized axonal regeneration (28).
Table 2
| Seddon | Sunderland | Structures disrupted | Prognosis |
|---|---|---|---|
| Neuropraxia | First degree | Myelin sheath | Good, full recovery expected immediately—2 weeks |
| Axonotmesis | Second degree | Axon | Good, full recovery expected at time frame of 1 mm/day |
| Third degree | Axon + endoneurium | Variable, recovery may be incomplete | |
| Fourth degree | Axon + endoneurium + perineurium | Poor, frequently with neuroma formation | |
| Neurotmesis | Fifth degree | Axon + endoneurium + perineurium + epineurium (and vaso nervorum) | Poor, frequently in discontinuity with end stump neuroma |
Mechanism of injury
As stated above, it has been reported that there are three primary mechanisms of BPI in contact athletes, including traction injury, direct force or compression, and cervical root compression via neck hyperextension or flexion. The traction injury is most commonly described as a downward force on ipsilateral shoulder and flexion of the neck towards the contralateral side of the injury as seen in Figure 1 (6). Location of the injury along the plexus may also vary based on the athletes position of the arm, neck, or the direction of an applied force (2). An arm adducted down at the side with a downward force may result in an upper trunk injury vs. an abducted arm pulled overhead may result in a lower trunk injury as the nerves typically fail in tension (2,5,27). Within contact sports, the arm is typically adducted, resulting in more frequent upper trunk injury of the brachial plexus compared to lower trunk injuries (6,7).
A frequent maneuver in football, rugby, wrestling, and sometimes ice hockey is the head-up, shoulder contact tackle in which contact is made with another player at the cervical-thoracic junction. These collisions may occur at a range of velocities, which may explain the predispositions of particular position players to BPI or neuropraxia events. Epidemiologic study of NFL players has demonstrated that running backs, linebackers, and defensive linemen are the most at risk position groups (7). It has been supposed that these groups are at higher risk than position groups that make regular contact on every single play, such as offensive linemen, due to the increased velocity with which the collisions occur. The inception of rules against head down or spear tackling in 1976 and targeting infractions in 2018 meant to discourage unsafe tackling techniques that increase axial loading of the cervical spine may have increased the contact made directly at the shoulder with shoulder depression and neck lateral flexion, consequentially causing traction of the brachial plexus (7,29,30). However, there is a notable decrease in the number of cervical spine injuries and concussions due to these rule changes with likely overall net positive effect on player safety (29-32).
Evaluation and diagnosis
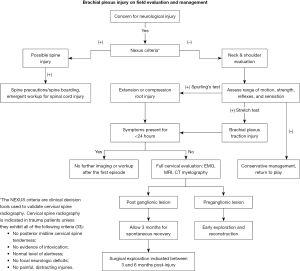
Diagnosis of a BPI following a contact injury is critical to safely return an athlete to play. The severity of nerve injury may influence the duration of symptoms, but frequently neuropraxia, axonotmesis, and neurotmesis may all present with unilateral upper extremity weakness, pain, often neuropathic in nature (i.e., burning, stinging, and radiating pain), and sensation changes. Bilateral upper extremity symptoms, lower extremity symptoms, pain and motor loss without sensation changes, neck pain, or concussion type symptoms (headache, nausea, coordination difficulties, double vision or light sensitivity, etc.) during on field or initial clinical evaluation may indicate more severe etiologies of upper extremity symptoms and warrant cautious evaluation, as shown in Figure 4 (7,31,33).
BPI is primarily a clinical diagnosis, with potential adjuncts such as EMG, nerve conduction studies (NCS), magnetic resonance imaging (MRI), and computed tomography (CT) myelogram to assist in nuanced evaluation. As previously stated, initial symptoms at the time of impact may include weakness, neuralgia, and sensory changes. The injury may evolve with delayed onset of some symptoms, such as resolution of initially present weakness or weakness not present initially, evolving at 1–3 days post-injury (8). With a classic stinger injury, all symptoms should resolve within minutes to hours. Some symptoms may persist in neuropraxic injuries, but should resolve within 2 weeks (6-8,10,28). An injury that persists beyond 2 weeks likely represents axonotmesis or neurotmesis, however, distinguishing these on clinical exam may be difficult, though important for treatment and prognostication purposes (5,6,8,10).
Electrodiagnostic testing may be maximally beneficial around this period due to the suspension of recognizable changes that may occur in the first 2 weeks as Wallerian degeneration occurs. NCS timing is crucial as the compound motor action potentials (CMAPs) and sensory neuron action potentials (SNAPs) deteriorate at different rates. CMAPs may become abnormal on day 2 or 3 following injury without reaching the nadir till day 7 and SNAPs may decrease on day 6 but not reach the nadir until day 10 (34,35). As previously mentioned, an intact sensory axon in a preganglionic injury may still produce a normal SNAP, thus an ill-timed electrodiagnostic test may lead to a misdiagnosis (24). EMG changes reflecting Wallerian degeneration via the effects on the hypersensitivity of the muscle groups resulting in decreased motor unit action potentials (MUAPs) and spontaneous fibrillations become evident around 10–14 days in proximal muscles and approximately 3–6 weeks in more distal musculature (22). These findings all represent axonal injury. Severe axonotmesis or complete neurotmesis would present with absent MUAPs, absent SNAPs, absent CMAPs, with only spontaneous muscle fibrillations on EMG (27,35,36).
If suspicion is high for root avulsion or pre-ganglionic injury, physical examination may be helpful in evaluating the proximal branches of the brachial plexus. As stated previously, the dorsal scapular nerve and long thoracic nerves are often intact in a post-ganglionic injury. Thus, in a patient who is unable to hold the shoulder protracted position utilizing their rhomboids or has evidence of scapular winging due to inability to fire the serratus anterior on attempts to elevate the shoulder, a root avulsion or pre-ganglionic injury should be suspected (2,5,22). MRI and CT myelogram have both been demonstrated to be helpful in detecting a pseudomeningocele, or traumatic outpouching of cerebrospinal fluid (CSF), as evidence of root avulsion. However, in the acute setting of the first 1–4 weeks, hematoma may block this outpouching of CSF and decrease sensitivity of these studies (2,22,36). MRI has been supposed to be the superior imaging modality as it has the additional advantage of evaluating more peripheral injuries of the plexus (22,37,38). Peripheral hematoma or edema may also inhibit sensitivity to detect injuries, and thus subacute imaging may be more helpful than within the first few days of injury (36).
Recurrent BPI or burners may warrant evaluation for cervical canal or foraminal stenosis. Meyer et al. in 1994 evaluated the Torg ratio in football players who had or had not experienced a stinger episode due to sport participation (39). The Torg ratio divides the width of the cervical canal on a lateral spine radiograph by the width of the cervical vertebral body (40). A significantly higher proportion of athletes who sustained a stinger episode had a Torg ratio of less than 0.8 (39). Further studies have delineated that those athletes who experience multiple stinger episodes had a statistically smaller Torg ratio than those who only had one episode, 0.75 vs. 0.87, respectively (20). Levitz et al. evaluated 55 football players who sustained recurrent or prolonged burner episodes and found that 71% of them had a positive Spurling sign on physical exam and 53% of them had evidence of cervical stenosis on imaging (19).
Return to sport
Return to play in athletes with burners or neuropraxic injuries is frequently possible within the same game, if not within the same week. Critical evaluation to rule out concomitant injury is necessary prior to return. Lamplot et al. (7) found that 6.51% of athletes with burners had a concomitant injury, shoulder injuries being the most common followed by cervical spine, and then concussions. National Emergency X-Radiography Utilization Study (NEXUS) criteria indicate cervical spine imaging in patients with focal neurologic deficit, midline spine tenderness, altered mental status, intoxication, or distracting injuries in the setting of spine trauma (41). NFL protocols mandate that all athletes diagnosed with a stinger or burner be evaluated with a concussion sideline assessment (31). In the absence of evidence of concomitant injury, a player should demonstrate full return to neurologic baseline and pain-free range of motion at the shoulder and the neck prior to return to play after BPI (6-8,10).
For some athletes returning from a neuropraxic injury and most returning from an axonotmesis or neurotmesis injury, initiation of a rehabilitation protocol for shoulder and neck strengthening should be initiated once there is evidence of return of neurologic function either clinically or on EMG (5,6,8,21). There is no standardized rehabilitation protocol that exists within the literature at this time. Proposed protocols, such as by Cramer in 1999, have outlined post-season recovery and rehabilitation protocols, however no randomized controlled trials exist for determination of effectiveness of prevention of repeat BPI or effect on long-term outcomes (42). The majority of these protocols call for a focus on range of motion of the cervical spine and shoulder girdle alongside strengthening of the paraspinal, cervical, and shoulder musculature (6,13,21,42). For prevention, a “Cowboy Collar” or neck roll to prevent hyperextension of the neck has been suggested (5,6,8,21,43). According to Levitz, as well as Reilly and Torg (19), reduction of hyperextension of the neck decreases compression of the nerve roots at the neuroforamen. These equipment adjuncts have been demonstrated by laboratory studies to significantly reduce hyperextension, however neck rolls, neck collars, or Cowboy Collars have not been shown to significantly reduce passive lateral flexion, a significant contributor to the tension mechanism of BPI (44,45). Evidence is limited primarily to case reports and case series, significantly reducing the amount of strength of recommendations, however in the absence of demonstrated harm and other prevention mechanisms, rehabilitation and equipment adjuncts are empirically recommended. Athletes with recurrent burners or persistent symptoms should undergo further evaluation as outlined in Figure 4 and discussed above.
Treatment and recovery
Treatment and recovery from BPI are dependent on the location of the injury and the severity of damage to the tissue. Almost all neuropraxia and some axonotmesis injuries will resolve with conservative management. The recovery timeline can vary, and while most burners resolve immediately, some neuropraxic injuries may take weeks to recover (8,10). Other axonotmesis injuries may require weeks to months depending on the length of the segment of axon that has been damaged. Axonal regeneration occurs at a rate of approximately 1 mm/day, and thus the time of recovery is variable (2,6,8,10). This variation and the relative rarity of severe BPI requiring surgical intervention can make treatment course delineation difficult and thus literature is limited primarily to case series and cohort studies.
In an effort to determine the efficacy of early exploration and decompression, Altaf et al. reported a case series of 13 rugby athletes who underwent exploration for BPIs. Six patients underwent decompression from fibrosis at the time of exploration. All of these patients had resolution of pain within 5 months of surgery and return of motor strength by 6 months (14). These authors hypothesized that hematoma at the time of injury leads to fibrosis formation and compression at the site of the injury. A general wait time of 3 months prior to surgical intervention for lesions in continuity is appropriate for allowing possible spontaneous recovery. EMG is helpful in assessing for signs of regeneration with elongated MUAPs or increasing recruitment being associated with regeneration (35). A window of 3–6 months has been supposed for surgical intervention as after 6 months muscular atrophy and fibrosis make intervention technically challenging with a low chance for success (2,35).
Neurotmesis frequently requires more direct and expedited surgical intervention given the discontinuity of the nerve and minimal potential for spontaneous regeneration due to ischemia. Pre-ganglionic injuries typically require immediate surgical intervention. The approach to reconstruction is determined by the location along the plexus with the goal of bridging the zone of injury via grafting or nerve transfers. Pre-ganglionic injury due to the discontinuity of the nerve from the spinal cord and motor cortex cell body is a contraindication to grafting as the zone of injury is at the level of the spinal cord and thus cannot be bridged. EMG/NCS should be used to evaluate possible nerve transfer candidates, with preference for donors being given to those nerves with normal electrodiagnostic findings (2,46). Additionally, EMG should evaluate the health of the target muscle or reinnervation, absence of spontaneous fibrillations or sharp waves at rest represents irreversible muscle fibrosis and less receptivity for reinnervation (35,47). Nerve transfers should be aimed at restoring function first for elbow flexion and secondly shoulder stability in patients with complete or upper trunk injuries (2,22).
Limitations and future directions
Limitations exist within the search parameters of this narrative review. This includes the possible bias toward contact sports in English-speaking countries due to language restrictions. Large sample size studies of neuropraxic injuries and small sample size studies of axonotmesis and neurotmesis injuries represent a discrepancy in knowledge about the less common, but more severe injuries. Additionally, there is far more literature available regarding tackling contact sports such as football and rugby due to the relative higher incidence, which may bias some understanding of possible mechanisms of injury. Additional studies are needed to explore these injuries in less common contact sports. Treatment of these injuries is largely still controversial and larger randomized and controlled studies are needed to further understand effective rehabilitation, discrete timing for intervention, and the specific indications for those interventions.
Conclusions
In conclusion, BPI in the contact athlete most commonly presents as spontaneously resolving low-grade neuropraxia. However, the potential for severe and potentially permanent axonal damage exists with the post-ganglionic upper trunk being the most commonly affected anatomic region. In post-ganglionic injuries, clinical diagnosis and differentiation of severity may be difficult and often EMG and MRI may aid in evaluation after a 2-week time period of observation. Surgical intervention is most effective at 3–6 months for post-ganglionic injuries, and as immediate as possible for pre-ganglionic injuries. Further studies are needed to expand the understanding of the appropriate evaluation and criteria for surgical intervention of injuries with failure to spontaneously recover.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Justin W. Arner) for the series “Care of the Contact Athlete’s Shoulder” published in Annals of Joint. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-24-67/rc
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-24-67/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-24-67/coif). The series “Care of the Contact Athlete’s Shoulder” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaiser R, Waldauf P, Ullas G, et al. Epidemiology, etiology, and types of severe adult brachial plexus injuries requiring surgical repair: systematic review and meta-analysis. Neurosurg Rev 2020;43:443-52. [Crossref] [PubMed]
- Wu KY, Spinner RJ, Shin AY. Traumatic brachial plexus injury: diagnosis and treatment. Curr Opin Neurol 2022;35:708-17. [Crossref] [PubMed]
- Tosti R, Rossy W, Sanchez A, et al. Burners, stingers, and other brachial plexus injuries in the contact athlete. Oper Tech Sports Med 2016;24:273-7. [Crossref]
- Starr HM Jr, Anderson B, Courson R, et al. Brachial plexus injury: a descriptive study of American football. J Surg Orthop Adv 2014;23:90-7. [Crossref] [PubMed]
- Koffler KM, Kelly JD 4th. Neurovascular trauma in athletes. Orthop Clin North Am 2002;33:523-34. vi. [Crossref] [PubMed]
- Armstrong R, McKeever T, Leavitt M, et al. Rehabilitation of brachial plexus injury in contact sport: Where are the data that underpin clinical management? A scoping review. PLoS One 2024;19:e0298317. [Crossref] [PubMed]
- Lamplot JD, Petit C, Lee R, et al. Epidemiology of Stingers in the National Football League, 2015-2019. Sports Health 2024;16:565-72. [Crossref] [PubMed]
- Clancy WG Jr, Brand RL, Bergfield JA. Upper trunk brachial plexus injuries in contact sports. Am J Sports Med 1977;5:209-16. [Crossref] [PubMed]
- Soldado F, Ghizoni MF, Bertelli J. Injury mechanisms in supraclavicular stretch injuries of the brachial plexus. Hand Surg Rehabil 2016;35:51-4. [Crossref] [PubMed]
- Nissen SJ, Laskowski ER, Rizzo TD Jr. Burner syndrome: recognition and rehabilitation. Phys Sportsmed 1996;24:57-64. [Crossref] [PubMed]
- Green J, Zuckerman SL, Dalton SL, et al. A 6-year surveillance study of “Stingers” in NCAA American Football. Res Sports Med 2017;25:26-36. [Crossref] [PubMed]
- Weinberg J, Rokito S, Silber JS. Etiology, treatment, and prevention of athletic “stingers”. Clin Sports Med 2003;22:493-500. viii. [Crossref] [PubMed]
- Belviso I, Palermi S, Sacco AM, et al. Brachial Plexus Injuries in Sport Medicine: Clinical Evaluation, Diagnostic Approaches, Treatment Options, and Rehabilitative Interventions. J Funct Morphol Kinesiol 2020;5:22. [Crossref] [PubMed]
- Altaf F, Mannan K, Bharania P, et al. Severe brachial plexus injuries in rugby. Injury 2012;43:272-3. [Crossref] [PubMed]
- Sallis RE, Jones K, Knopp W. Burners. Phys Sportsmed 1992;20:47-55. [Crossref] [PubMed]
- Beaulieu-Jones BR, Rossy WH, Sanchez G, et al. Epidemiology of Injuries Identified at the NFL Scouting Combine and Their Impact on Performance in the National Football League: Evaluation of 2203 Athletes From 2009 to 2015. Orthop J Sports Med 2017;5:2325967117708744. [Crossref] [PubMed]
- Estwanik JJ 3rd, Bergfeld JA, Collins HR, et al. Injuries in Interscholastic Wrestling. Phys Sportsmed 1980;8:111-21. [Crossref] [PubMed]
- Hartley RA, Kordecki ME. Rehabilitation of chronic brachial plexus neuropraxia and loss of cervical extension in a high school football player: a case report. Int J Sports Phys Ther 2018;13:1061-72. [Crossref] [PubMed]
- Levitz CL, Reilly PJ, Torg JS. The pathomechanics of chronic, recurrent cervical nerve root neurapraxia. The chronic burner syndrome. Am J Sports Med 1997;25:73-6. [Crossref] [PubMed]
- Castro FP Jr, Ricciardi J, Brunet ME, et al. Stingers, the Torg ratio, and the cervical spine. Am J Sports Med 1997;25:603-8. [Crossref] [PubMed]
- Reilly PJ, Torg JS. Athletic injury to the cervical nerve roots and brachial plexus. Oper Tech Sports Med 1993;1:231-5. [Crossref]
- Shin AY, Spinner RJ, Steinmann SP, et al. Adult traumatic brachial plexus injuries. J Am Acad Orthop Surg 2005;13:382-96. [Crossref] [PubMed]
- Saliba S, Saliba EN, Pugh KF, et al. Rehabilitation considerations of a brachial plexus injury with complete avulsion of c5 and c6 nerve roots in a college football player: a case study. Sports Health 2009;1:370-5. [Crossref] [PubMed]
- Levin KH, Wilbourn AJ, Maggiano HJ. Cervical rib and median sternotomy-related brachial plexopathies: a reassessment. Neurology 1998;50:1407-13. [Crossref] [PubMed]
- Gutkowska O, Martynkiewicz J, Urban M, et al. Brachial plexus injury after shoulder dislocation: a literature review. Neurosurg Rev 2020;43:407-23. [Crossref] [PubMed]
- Denny-Brown D, Doherty MM. Effects of transient stretching of peripheral nerve. Arch Neurol Psychiatry 1945;54:116-29. [Crossref]
- Sakellariou VI, Badilas NK, Mazis GA, et al. Brachial plexus injuries in adults: evaluation and diagnostic approach. ISRN Orthop 2014;2014:726103. [Crossref] [PubMed]
- Chhabra A, Ahlawat S, Belzberg A, et al. Peripheral nerve injury grading simplified on MR neurography: As referenced to Seddon and Sunderland classifications. Indian J Radiol Imaging 2014;24:217-24. [Crossref] [PubMed]
- Chao S, Pacella MJ, Torg JS. The pathomechanics, pathophysiology and prevention of cervical spinal cord and brachial plexus injuries in athletics. Sports Med 2010;40:59-75. [Crossref] [PubMed]
- Heck JF, Clarke KS, Peterson TR, et al. National Athletic Trainers’ Association Position Statement: Head-Down Contact and Spearing in Tackle Football. J Athl Train 2004;39:101-11. [PubMed]
- Ellenbogen RG, Batjer H, Cardenas J, et al. National Football League Head, Neck and Spine Committee’s Concussion Diagnosis and Management Protocol: 2017-18 season. Br J Sports Med 2018;52:894-902. [Crossref] [PubMed]
- Mack CD, Solomon G, Covassin T, et al. Epidemiology of Concussion in the National Football League, 2015-2019. Sports Health 2021;13:423-30. [Crossref] [PubMed]
- Collins NC, McKenzie JV. The NEXUS criteria: do they stand the test of time? Eur J Emerg Med 2013;20:58-60. [Crossref] [PubMed]
- Ferrante MA, Wilbourn AJ. Electrodiagnostic approach to the patient with suspected brachial plexopathy. Neurol Clin 2002;20:423-50. [Crossref] [PubMed]
- Dy CJ, Colorado BS, Landau AJ, et al. Interpretation of Electrodiagnostic Studies: How to Apply It to the Practice of Orthopaedic Surgery. J Am Acad Orthop Surg 2021;29:e646-54. [Crossref] [PubMed]
- O’Shea K, Feinberg JH, Wolfe SW. Imaging and electrodiagnostic work-up of acute adult brachial plexus injuries. J Hand Surg Eur Vol 2011;36:747-59. [Crossref] [PubMed]
- Yeow YJ, Yeow KM, Su IH, et al. Predicting Healthy C5 Spinal Nerve Stumps Eligible for Grafting with MRI, Tinel Test, and Rhomboid Electromyography: A Retrospective Study of 295 Consecutive Brachial Plexus Surgeries. Radiology 2021;300:141-51. [Crossref] [PubMed]
- Doi K, Otsuka K, Okamoto Y, et al. Cervical nerve root avulsion in brachial plexus injuries: magnetic resonance imaging classification and comparison with myelography and computerized tomography myelography. J Neurosurg 2002;96:277-84. [PubMed]
- Meyer SA, Schulte KR, Callaghan JJ, et al. Cervical spinal stenosis and stingers in collegiate football players. Am J Sports Med 1994;22:158-66. [Crossref] [PubMed]
- Tierney RT, Maldjian C, Mattacola CG, et al. Cervical Spine Stenosis Measures in Normal Subjects. J Athl Train 2002;37:190-3. [PubMed]
- Hoffman JR, Wolfson AB, Todd K, et al. Selective cervical spine radiography in blunt trauma: methodology of the National Emergency X-Radiography Utilization Study (NEXUS). Ann Emerg Med 1998;32:461-9. [Crossref] [PubMed]
- Cramer CR. A reconditioning program to lower the recurrence rate of brachial plexus neurapraxia in collegiate football players. J Athl Train 1999;34:390-6. [PubMed]
- Speer KP, Bassett FH 3rd. The prolonged burner syndrome. Am J Sports Med 1990;18:591-4. [Crossref] [PubMed]
- Gorden JA, Straub SJ, Swanik CB, et al. Effects of Football Collars on Cervical Hyperextension and Lateral Flexion. J Athl Train 2003;38:209-15. [PubMed]
- Stuber K. Cervical collars and braces in athletic brachial plexus injury and excessive cervical motion prevention: a review of the literature. J Can Chiropr Assoc 2005;49:216-22. [PubMed]
- Schreiber JJ, Feinberg JH, Byun DJ, et al. Preoperative donor nerve electromyography as a predictor of nerve transfer outcomes. J Hand Surg Am 2014;39:42-9. [Crossref] [PubMed]
- Kraft GH. Fibrillation potential amplitude and muscle atrophy following peripheral nerve injury. Muscle Nerve 1990;13:814-21. [Crossref] [PubMed]
Cite this article as: Windmueller RA, Gbayisomore OO, Mead RL, Desai MJ, Bowman EN. Brachial plexus injuries in the contact athlete: a narrative review. Ann Joint 2025;10:18.


