
Recurrent peroneal tendon dislocation—the current concept of management
Introduction
The peroneus consists of two muscles: the peroneus longus (PL) and peroneus brevis (PB). The PL originates from the upper 2/3 of the fibula and stops at the second metatarsal floor and the medial cuneiform bone, while the PB originates from the lower 2/3 part of the fibula and stops at the fifth metatarsal floor. Peroneus tendons are susceptible to injury at anatomical sites of altered tendon pathway or where a tendon sheath is present. Specifically, they include the (I) posterior portion of the ankle lateral malleolus [superior peroneal retinaculum (SPR)]; (II) peroneal tendon pulley [inferior peroneal retinaculum (IPR)]; and (III) cuboid tunnel. This paper describes the evaluation and management of peroneal tendon dislocation (PTD), a disorder that occurs in (I) the posterior portion of the ankle lateral malleolus.
PTD
Etiology
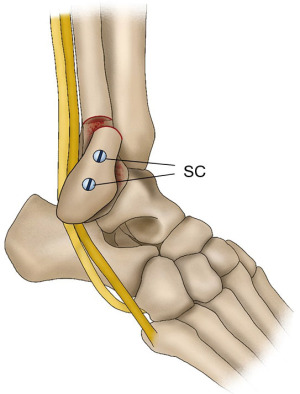
PTD is a rare trauma that accounts for 0.3–0.5% of all ankle traumas (1-3). PTD generally occurs after dorsiflexion in a slightly everted foot, followed by strong contraction of the peroneal muscles during sports including skiing, ice dancing, soccer, basketball, rugby, and gymnastics. Acute PTD is characterized by swelling, pain, and tenderness around the lateral malleolus. Thus, it is often misdiagnosed as lateral ankle sprain, a common sports injury (4,5). Owing to misdiagnosis as a lateral ankle sprain and other factors, many patients with PTD progress to recurrent PTD (RPTD). In the posterior part of the ankle lateral malleolus, the PL and PB tendons run within the same tendon sheath, changing their running direction at the tip of the fibula and head anteriorly. The tendons are prevented from dislocation by the fibrocartilaginous ridge (FCR), a structure similar to an articular labrum in the shoulder joint, and the SPR prevents the peroneal tendons from dislocation (Figure 1). The PL tendon is often dislocated as the PB tendon is stuck in a concave depression on the fibular side, while the PL tendon passes through the dorsal (superficial) side. At this level, the peroneal tendons are surrounded by the SPR, which attaches to the FCR, and the lateral surface of the fibula and protects the tendons against dislocation. When these structures including the SPR and FCR, fail, PTD occurs. Moreover, if these structures detached from the fibula do not heal in good alignment with the fibula, they create a space on the fibula surface, called a ‘pseudo-pouch’, leading to RPTD.

Several anatomical variations in the peroneal muscles and lateral malleolus of the ankle that may play an important role in PTD onset have been reported. The shape of the retromalleolar groove is considered an anatomical feature of the PTD. Saupe et al. (6) classified the retromalleolar groove as concave (28%), flat (43%), convex (18%), or irregular (11%), based on magnetic resonance imaging (MRI). Ozbag et al. (7) reported that the presence of concave-type (67%) retromalleolar grooves was significantly related to RPTD in a cadaveric study. However, a recent report (8) revealed that the shape of the retromalleolar groove was not related to the PTD based on computed tomography (CT). The reason behind these differences between these results is the anatomical structure of the retromalleolar groove. The concave structure of the retromalleolar groove is not made up of bone alone but consists of a combination of cartilage components, including the FCR (9). Particularly, the components of the fibula have been found to be increasingly cartilaginous toward the tip of the fibula. Therefore, the bone component alone had no significant relationship with PTD, but a significant relationship was observed in the cadaver study that included the cartilage. Soft tissue overstuffing within the peroneal tendon sheath is another anatomical factor associated with PTD. The presence of accessory peroneal muscles including the peroneus quartus and peroneus quintus muscles (10) and low-lying muscle belly (LLMB) of the PB are soft tissue variants that are proposed to be possible causes of overstaffing within the peroneal tendon sheath. A recent MRI study (8) revealed that a low-lying PB muscle belly and large volume in the retromalleolar space were significantly associated with PTD. In this study, the soft tissue volume in the retromalleolar space was used as an anatomical marker of PTD risk.
Classification
Eckert et al. (11) classified PTDs into three types according to the type of injury to the FCR and SPR, while Oden et al. (12) added type 4 with the peroneal tendon dislocated due to rupture of the SPR (Figure 2):
- Type 1: dislocation occurs when the SPR continuously detaches from the FCR and fibula with the fibular anterior periosteum. This type is the most reported one, accounting for 51% of all cases.
- Type 2: this is a dislocation with the FRC, SPR, and periosteum on the anterior surface of the fibula collectively avulsed and dislocated. This type is reported in 33% of the cases.
- Type 3: this is a type of avulsion fracture of the bone of the anterior surface of the fibula with combined FCR and SPR, with an incidence rate of 13%.
- Type 4: this is a rare type with the SPR itself is ruptured, later added by Oden et al. (12); its frequency is unknown.

Diagnosis
In cases with acute dislocation, patients complain of pain around the ankle lateral malleolus, as in an inversion ankle sprain; however, the main tender point in an inversion ankle sprain is anterior to the ankle lateral malleolus, whereas in cases with PTD, the tender point is posterior to the ankle lateral malleolus. Patients with RPTD may experience repetitive tendon dislocation during sports activities or in daily life and complain of pain and instability. Some patients may be able to reproduce the dislocation on their own but often cannot reproduce the dislocation in the clinic. Some patients describe the sensation of tendon dislocation as a sprain. Although in most cases of PTD, no abnormal findings are observed on a simple radiograph or CT scan, two-way imaging should be performed to rule out other conditions including fractures. Only Type 3 dislocations of avulsion fractures with SPR from the fibula can be observed on simple radiography or CT. MRI may also point to variations including the fourth peroneal tendon and/or peroneal tendon tears. Moreover, MRI can sometimes reveal abnormalities such as a pseudo-pouch, but not in all cases. Ultrasonography allows dynamic observation of tendon movement, and the presence or absence of a pseudo-pouch can be observed by injecting a liquid (saline, local anesthetic, etc.) into the tendon sheath (Figure 3). Ultrasonography is very useful for diagnosis because it can be performed in real time in the examination room, and if a pseudo-pouch is observed, PTD can be diagnosed.

Conservative treatment
Previous studies have reported the results of conservative treatment for acute PTD (11,13). A systematic review by Bakker et al. (5) reported that a 4–6-week below-knee plaster cast prevented recurrent dislocation in 62–83% of the patients. Moreover, in their review, acute PTD treated with tape for 3 weeks resulted in only a 40% success rate. Therefore, a below-knee cast is preferable for tape fixation. If conservative treatment fails and re-dislocation occurs, surgical therapy is required. Some authors advocate surgical repair even in acute PTD cases because of the high failure rate of conservative treatment, especially in athletes (2).
Operative treatment
Surgery is usually performed when conservative treatment fails to resolve the occurrence of recurrent dislocations. As noted above, several studies recommend surgery, even for initial PTD in athletes. Surgical treatment is divided into three main categories, osteotomy, soft tissue, and groove deepening techniques (14).
Osteotomy techniques involves partial or complete fibular osteotomy with posterior displacement or rotation of the more lateral fragment to serve as a physical barrier to prevent anterior subluxation of the tendons (15,16). Kelly initially described a partial-thickness distal fibular osteotomy, rotated posteriorly to deepen the fibular groove (15) (Figure 4). Several procedures have been devised to modify Kelly’s technique (17). Osteotomy techniques have the advantage of stabilizing the peroneal tendons more firmly than the soft tissue techniques described below. However, the osteotomy procedure has several shortcomings such as fractures, delayed/nonunion of bony fragments, postoperative tendon irritation, and tendon adherence to the underlying bone (18,19).
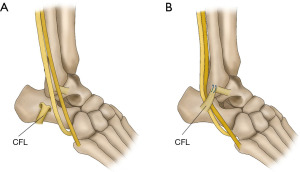
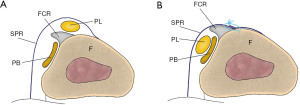
The soft tissue procedure consists of rerouting, SPR reconstruction, and SPR reattachment. The most used rerouting procedure involves the use of the calcaneofibular ligament (CFL). Platzgummer’s method involves separation of the CFL near the attachment of the fibula and re-suturing it through the peroneus tendon underneath it (20,21) (Figure 5). Pöll and Duijfjes (22) described osteotomy of the distal fibula, including the CFL, and Pozo and Jackson (23) described osteotomy of the CFL from the calcaneal attachment and passing the peroneus tendon underneath (Figure 6). Martens et al. (24) described a method in which the peroneus tendons were divided, passed beneath the CFL, and re-sutured (Figure 7). Although no re-dislocation was observed, complications such as peroneal nerve injury and ankle stiffness were reported in 62% of the cases using this method (25). Rerouting is less common today because it is not anatomic and requires the invasion of normal tissues including the CFL. SPR has been performed using the Achilles tendon (13), plantaris (26), and PB (27). These procedures also require the invasion of normal tissues. McGarvey and Clanton reported a 19% complication rate using this technique (25). SPR reconstruction may be an option when the SPR is absent; however, in many cases, the SPR is retained and can be used. Thus, the main soft tissue procedure is SPR reattachment, which is a method for reconstructing anatomically normal structures (11,28-31) (Figure 8).


In a study comparing the osteotomy and SPR reattachment results, Tomihara et al. (32) revealed that SPR reattachment provided similar or slightly better clinical outcomes with fewer complications than osteotomy. In their report, the rate of return to sports was higher, and the duration to return to sports was significantly shorter in the SPR reattachment group than in the osteotomy group, and they recommended SPR reattachment for athletes. A recent review comparing osteotomy and soft tissue procedures reported no differences in the revision rates between the two techniques (14). This study concluded that soft tissue procedures offer a satisfactory method of treating RPTD without any additional risk of reoperation compared with osteotomy techniques, which have potentially greater complication rates.
Groove deepening techniques are used to stabilize peroneal tendons and prevent dislocation by deepening the groove that receives the peroneal tendon on the posterior wall of the fibula at ankle level (33-37). Because the superficial layer of the retromalleolar groove is covered with fibrocartilage for smooth gliding of the peroneal tendon, some procedures have attempted to preserve these structures rather than simply shaving the groove deeper. For example, in the technique of Walther et al. (37), the cancellous bone behind the groove was removed with a 3.5-mm drill and the media and lateral border of the retromalleolar groove were broken using an osteotome to prevent fracture during impaction. Subsequently, the retromalleolar groove is affected (Figure 9). This method is often used in combination with SPR. A recent systematic review found that combining SPR repair and retromalleolar groove deepening provides a significantly higher return to sports than SPR repair alone (2). They concluded that an additional groove deepening procedure is recommended for athletes in this review paper. However, a recent study that examined anatomical features using CT and MRI reported that PTD is more affected by the amount of soft tissue, such as the muscle belly, in the peroneal tendon sheath than by the bony morphology of the retromalleolar groove (8). This anatomical study suggests that it is not always necessary to deepen the groove. Further research is warranted to determine how to control the excessive soft tissue volume, in which groove deepening is essential.

Soft tissue procedures such as SPR reattachment and groove deepening have been performed using an endoscope. In general, tendoscopy has advantages in terms of less postoperative pain and fewer complications than open procedures (38). Scholten et al. (39) reported a tendoscopic fibular groove-deepening procedure for RPTD. This method is very simple but has the disadvantage that the fibrocartilage on the retromalleolar groove must be removed because the tendon groove is simply shaved under the tendoscope. However, some reports (40-42) have reported tendoscopic SPR reattachment for RPTD. There are two methods for tendoscopic SPR reattachment: the single-raw and double-raw methods with suture bridges, as in the case of arthroscopic rotator cuff repair. The double-raw method has the advantage of increasing the healing rate by increasing the contact area between the SPR and the bone; however, it is more complicated than the single-raw method. Moreover, because there is little subcutaneous fat around this area, knot irritation is often a problem, and techniques using knotless anchors have been reported (3) (Figure 10). Although these tendoscopic methods have disadvantages compared with conventional methods, such as the need to learn arthroscopic techniques and longer operating times, they have been reported to have advantages in terms of cosmetics and the possibility of early return to sports (43).
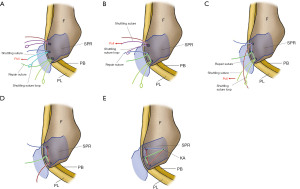
Intrasheath subluxation
Intrasheath subluxation of the peroneal tendon is a unique type of dislocation. This is a dislocation with the positional relationship of the PB and PL tendons is interchanged and the SPR is intact (44). Raikin et al. reported that 14 (25%) of 57 cases of peroneal tendon snapping in the retromalleolar groove were intrasheath subluxations. Ultrasonography can capture tendon dynamics in real time and is useful in diagnosing this condition (Figure 11). Raikin et al. classified intrasheath subluxation into two categories (Figure 12). Type A involves PL tendon subluxation around an intact PB tendon while it remains within the peroneal groove and sheath. Type B involves PL tendon subluxation through an associated longitudinal split tear of the PB tendon while remaining within the peroneal groove and sheath. The condition is often asymptomatic and requires no specific treatment. However, surgery is indicated if the patient becomes symptomatic and resists conservative treatment. Favorable results can be obtained by incising the superior peroneal muscle branch, excising the synovium within the tendon sheath, and deepening the tendon groove, followed by repair of the SPR (36). In cases complicated by longitudinal rupture of the PB tendon (Type B), suturing of the torn area or tubularization of the remaining tendon for partial resection of the degenerated tendon is performed.


Conclusions
Although PTD is not a frequent sports injury such as ankle sprain, it is often encountered in clinical practice. In cases of acute PTD, the injured limb position and site of pain are the points of differentiation from ankle sprains. Ultrasonography is useful for diagnosis. Most RPTD cases form a pseudo-pouch; however, confirmation of its presence on imaging is difficult unless fluid accumulation is observed as well. Thus, a definitive diagnosis can be made if a pseudo-pouch is identified after fluid injection into the peroneal tendon sheath. Ultrasonography is also useful in cases of intrasheath dislocation with the long and brevis peroneal tendons observed in real time. Although conservative treatment is recommended during the acute phase, repetitive dislocations often occur, requiring surgery. Although various surgical methods have been reported, SPR reattachment, a soft tissue procedure with anatomical repair, is the most used method.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-24-10/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-24-10/coif). A.N. serves as an unpaid editorial board member of Annals of Joint from August 2023 to July 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roth JA, Taylor WC, Whalen J. Peroneal tendon subluxation: the other lateral ankle injury. Br J Sports Med 2010;44:1047-53. [Crossref] [PubMed]
- van Dijk PA, Gianakos AL, Kerkhoffs GM, et al. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc 2016;24:1155-64. [Crossref] [PubMed]
- Nishimura A, Nakazora S, Senga Y, et al. Knotless Tendoscopic Peroneal Retinaculum Repair Technique for Recurrent Peroneal Tendon Dislocation. Arthrosc Tech 2022;11:e1395-401. [Crossref] [PubMed]
- Zammit J, Singh D. The peroneus quartus muscle. Anatomy and clinical relevance. J Bone Joint Surg Br 2003;85:1134-7. [Crossref] [PubMed]
- Bakker D, Schulte JB, Meuffels DE, et al. Non-operative treatment of peroneal tendon dislocations: A systematic review. J Orthop 2020;18:255-60. [Crossref] [PubMed]
- Saupe N, Mengiardi B, Pfirrmann CW, et al. Anatomic variants associated with peroneal tendon disorders: MR imaging findings in volunteers with asymptomatic ankles. Radiology 2007;242:509-17. [Crossref] [PubMed]
- Ozbag D, Gumusalan Y, Uzel M, et al. Morphometrical features of the human malleolar groove. Foot Ankle Int 2008;29:77-81. [Crossref] [PubMed]
- Nishimura A, Nakazora S, Senga Y, et al. Anatomic Features of Patients With Recurrent Peroneal Tendon Dislocation. Am J Sports Med 2023;51:1312-8. [Crossref] [PubMed]
- Kumai T, Benjamin M. The histological structure of the malleolar groove of the fibula in man: its direct bearing on the displacement of peroneal tendons and their surgical repair. J Anat 2003;203:257-62. [Crossref] [PubMed]
- Yammine K. The accessory peroneal (fibular) muscles: peroneus quartus and peroneus digiti quinti. A systematic review and meta-analysis. Surg Radiol Anat 2015;37:617-27. [Crossref] [PubMed]
- Eckert WR, Davis EA Jr. Acute rupture of the peroneal retinaculum. J Bone Joint Surg Am 1976;58:670-2. [Crossref] [PubMed]
- Oden RR. Tendon injuries about the ankle resulting from skiing. Clin Orthop Relat Res 1987;63-9. [Crossref] [PubMed]
- Escalas F, Figueras JM, Merino JA. Dislocation of the peroneal tendons. Long-term results of surgical treatment. J Bone Joint Surg Am 1980;62:451-3. [Crossref] [PubMed]
- Yasui Y, Vig KS, Tonogai I, et al. Incidence of reoperation and wound dehiscence in patients treated for peroneal tendon dislocations: comparison between osteotomy versus soft tissue procedures. Knee Surg Sports Traumatol Arthrosc 2018;26:897-902. [Crossref] [PubMed]
- Kelly RE. An operation for chronic dislocation of the peroneal tendons. British Journal of Surgery 1919;7:502-4. [Crossref]
- Zhenbo Z, Jin W, Haifeng G, et al. Sliding fibular graft repair for the treatment of recurrent peroneal subluxation. Foot Ankle Int 2014;35:496-503. [Crossref] [PubMed]
- Micheli LJ, Waters PM, Sanders DP. Sliding fibular graft repair for chronic dislocation of the peroneal tendons. Am J Sports Med 1989;17:68-71. [Crossref] [PubMed]
- Beck E. Operative treatment of recurrent dislocation of the peroneal tendons. Arch Orthop Trauma Surg (1978) 1981;98:247-50. [Crossref] [PubMed]
- Larsen E, Flink-Olsen M, Seerup K. Surgery for recurrent dislocation of the peroneal tendons. Acta Orthop Scand 1984;55:554-5. [Crossref] [PubMed]
- Platzgummer H. On a simple procedure for the operative therapy of habitual peroneal tendon luxation. Arch Orthop Unfallchir 1967;61:144-50. [Crossref] [PubMed]
- Steinböck G, Pinsger M. Treatment of peroneal tendon dislocation by transposition under the calcaneofibular ligament. Foot Ankle Int 1994;15:107-11. [Crossref] [PubMed]
- Pöll RG, Duijfjes F. The treatment of recurrent dislocation of the peroneal tendons. J Bone Joint Surg Br 1984;66:98-100. [Crossref] [PubMed]
- Pozo JL, Jackson AM. A rerouting operation for dislocation of peroneal tendons: operative technique and case report. Foot Ankle 1984;5:42-4. [Crossref] [PubMed]
- Martens MA, Noyez JF, Mulier JC. Recurrent dislocation of the peroneal tendons. Results of rerouting the tendons under the calcaneofibular ligament. Am J Sports Med 1986;14:148-50. [Crossref] [PubMed]
- McGarvey W, Clanton TO. Peroneal tendon dislocations. Foot and Ankle Clinics 1996;1:325-42. [Crossref]
- Miller JW. Dislocation of peroneal tendons--a new operative procedure. A case report. Am J Orthop 1967;9:136-7. [PubMed]
- Stein RE. Reconstruction of the superior peroneal retinaculum using a portion of the peroneus brevis tendon. A case report. J Bone Joint Surg Am 1987;69:298-9. [Crossref] [PubMed]
- Adachi N, Fukuhara K, Tanaka H, et al. Superior retinaculoplasty for recurrent dislocation of peroneal tendons. Foot Ankle Int 2006;27:1074-8. [Crossref] [PubMed]
- Das De S, Balasubramaniam P. A repair operation for recurrent dislocation of peroneal tendons. J Bone Joint Surg Br 1985;67:585-7. [Crossref] [PubMed]
- Lui TH. Endoscopic peroneal retinaculum reconstruction. Knee Surg Sports Traumatol Arthrosc 2006;14:478-81. [Crossref] [PubMed]
- Mason RB, Henderson JP. Traumatic peroneal tendon instability. Am J Sports Med 1996;24:652-8. [Crossref] [PubMed]
- Tomihara T, Shimada N, Yoshida G, et al. Comparison of modified Das De procedure with Du Vries procedure for traumatic peroneal tendon dislocation. Arch Orthop Trauma Surg 2010;130:1059-63. [Crossref] [PubMed]
- Kollias SL, Ferkel RD. Fibular grooving for recurrent peroneal tendon subluxation. Am J Sports Med 1997;25:329-35. [Crossref] [PubMed]
- Maqdes A, Steltzlen C, Pujol N. Endoscopic fibular groove deepening for stabilisation of recurrent peroneal tendons instability in a patient with open physes. Knee Surg Sports Traumatol Arthrosc 2017;25:1925-8. [Crossref] [PubMed]
- Porter D, McCarroll J, Knapp E, et al. Peroneal tendon subluxation in athletes: fibular groove deepening and retinacular reconstruction. Foot Ankle Int 2005;26:436-41. [Crossref] [PubMed]
- Raikin SM. Intrasheath subluxation of the peroneal tendons. Surgical technique. J Bone Joint Surg Am 2009;91:146-55. [Crossref] [PubMed]
- Walther M, Morrison R, Mayer B. Retromalleolar groove impaction for the treatment of unstable peroneal tendons. Am J Sports Med 2009;37:191-4. [Crossref] [PubMed]
- Monteagudo M, Maceira E, Martinez de Albornoz P. Foot and ankle tendoscopies: current concepts review. EFORT Open Rev 2016;1:440-7. [Crossref] [PubMed]
- Scholten PE, Breugem SJ, van Dijk CN. Tendoscopic treatment of recurrent peroneal tendon dislocation. Knee Surg Sports Traumatol Arthrosc 2013;21:1304-6. [Crossref] [PubMed]
- Miyamoto W, Takao M, Miki S, et al. Tendoscopic Repair of the Superior Peroneal Retinaculum via 2 Portals for Peroneal Tendon Instability. Foot Ankle Int 2015;36:1243-50. [Crossref] [PubMed]
- Lui TH, Li CCH. Endoscopic Superior Peroneal Retinaculum Reconstruction Using Q-FIX MINI Suture Anchor. Arthrosc Tech 2023;12:e233-40. [Crossref] [PubMed]
- Michels F, Jambou S, Guillo S, et al. Endoscopic treatment of intrasheath peroneal tendon subluxation. Case Rep Med 2013;2013:274685. [Crossref] [PubMed]
- Nishimura A, Kato K, Nakazora S, et al. Tendoscopic peroneal retinaculum repair for recurrent peroneal tendon dislocation enables earlier return to sports than the open procedure. Knee Surg Sports Traumatol Arthrosc 2020;28:3318-23. [Crossref] [PubMed]
- Raikin SM, Elias I, Nazarian LN. Intrasheath subluxation of the peroneal tendons. J Bone Joint Surg Am 2008;90:992-9. [Crossref] [PubMed]
Cite this article as: Nishimura A, Fujikawa Y, Senga Y, Nakazora S, Konno C, Sudo A. Recurrent peroneal tendon dislocation—the current concept of management. Ann Joint 2024;9:40.


