
Changes of peripheral blood α-L-fucosidase activity in patients with rheumatoid arthritis: a cross-sectional study
Highlight box
Key findings
• Abnormal changes of α-L-fucosidase (AFU) activity may lead to disturbances in glucose and lipid metabolism.
What is known and what is new?
• Rheumatoid arthritis (RA) cannot be completely cured at present, which seriously affects the quality of life of patients.
• In summary, changes of peripheral blood AFU activity might be associated with progression of disease in RA patients.
What is the implication, and what should change now?
• The present study put forward a potential correlation between abnormal the changes of AFU activity in RA patients, in order to fully understand the specific mechanism of AFU in RA patients, which is benefit to reasonable application of AFU evaluation as a screen and monitoring tool.
Introduction
Rheumatoid arthritis (RA), a systemic autoimmune disease with approximately 1% prevalent population worldwide, is characterized by chronic synovial joint inflammation, overgrowth of synoviocytes, progressive erosions and cartilage destruction, which the etiology is still unclear (1,2). Life expectancy in RA patients is significantly short than healthy people, compared with the general population, patients with RA experience a 70% increased fatality rate, of which approximately 50% cases are due to cardiovascular disease (3-5).
Moreover, RA cannot be completely cured at present, which seriously affects the quality of life of patients (6). Clinically, it was found that the risk of concurrent diabetes and cardiovascular disease in patients with RA was significantly higher than that of the general population, and the proportion of metabolic syndrome was higher, which affected the prognosis of patients (7). Therefore, the clinical features of metabolic abnormalities of RA are needed to be explored to explain its pathogenesis and prognosis.
α-L-fucosidase (AFU) is a lysosomal acid hydrolase that was first discovered in cytolysosomes and is mainly involved in the hydrolysis of fucose-containing carbohydrate complexes (8). Its basic physiological function is to catalyze the catabolism of fucosyl oligosaccharides, glycoproteins, glycopeptides, glycosides, and is widely present in various tissues, cells and body fluids in the human body (9). Numerous studies have confirmed that AFU is a marker enzyme for diagnosing primary hepatic cancer (PHC) (10,11). In addition, it has been reported in the literature that serum AFU concentrations also increase in patients with acute and chronic liver disease, kidney disease, diabetes, stomach cancer, and pancreatic cancer (12). The relationship between AFU and autoimmune rheumatic disease was also discussed. For example, Endreffy et al. reported that AFU was associated with chronic inflammatory pathologies in a pediatric cohort, suggesting that AFU is a biomarker of chronic inflammation and autoimmunity (13); moreover, alterations in plasma level of AFU were significantly associated with the development of Sjögren’s syndrome, and the preliminary result should encourage further research on AFU as a possible differential biomarker for Sjögren’s syndrome (14). A prior study proposed that the immune function of AFU may be a diagnostic marker of rheumatic disorders. However, specific mechanism has not been discussed (15).
The development of RA requires the involvement of glucose and lipid metabolism. For example, Arias-de la Rosa et al. proved that metabolic disturbances associated with RA, and it is depended on the degree of inflammation and identify inflammation of adipose tissue (16); moreover, Ye et al. proved that RA patients should be evaluated early for the presence of IR to reduce the risk of metabolic diseases (17). Accordingly, the primary purpose of this study was to investigate the role of the changes of peripheral blood AFU activity in glucose and lipid metabolism in RA patients. We present this article in accordance with the TRIPOD reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-50/rc).
Methods
Study population
A cross-sectional study was performed using total of 96 patients with RA admitted to the Second Affiliated Hospital of Nantong University from February, 2018 to November, 2020 were enrolled in this study. There were 20 males and 76 females, with an average age of 59.62 years. Another 94 age-matched healthy volunteers served as a control group (Table 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of The Second Affiliated Hospital of Nantong University (No. 083467820). All the patients voluntarily participated in the study and informed consent was obtained before enrollment.
Table 1
| Characteristics | Healthy control (n=94) | Rheumatoid arthritis (n=96) | P value |
|---|---|---|---|
| Age (years) | 59.98±14.73 | 59.62±13.44 | >0.05 |
| Gender (male/female) | 26/68 | 20/76 | >0.05 |
| WBC (109/L) | 6.04±1.94 | 6.70±2.61 | <0.01 |
| Neutrophil (%) | 57.58±9.87 | 69.72±9.90 | <0.0001 |
| Lymphocytes (%) | 21.73±8.26 | 32.43±8.84 | <0.0001 |
| hs-CRP (mg/dL) | 20.85±57.30 | 26.16±41.28 | <0.0001 |
Data are presented as n or mean ± standard deviation. WBC, white blood cell; hs-CRP, high-sensitivity C-reactive protein.
Inclusion and exclusion criteria
The inclusion criteria were: (I) ≥18 years old; (II) patients who are diagnosed with the RA classification criteria revised by the American College of Rheumatology (ACR) in 1987 or the ACR/European League Against Rheumatism (EULAR) revision in 2010 (18,19).
Patients were excluded as follows: (I) patients with liver and kidney dysfunction [alanine transaminase (ALT) or aspartate transaminase (AST) over 1.5 times of the upper limit of normal value, creatinine or urea nitrogen over the upper limit of normal value]; (II) patients with thyroid or parathyroid gland disease; (III) patients with malignant tumors; (IV) patients with long-term bed rest (patients in bend for at least 3 months); (V) patients with long-term use of calcium and other drugs that affect bone density (glucocorticoids, anticoagulants, thyroid hormone, proton pump inhibitor, antiepileptic drugs, aromatase inhibitors, protease inhibitors, thiazolidinediones and anti-cancer drugs); (VI) patients with other rheumatic diseases; (VII) patients with severe hypertension (systolic blood pressure greater than 180 mmHg or diastolic blood pressure greater than 110 mmHg) and other metabolic diseases.
Assay methods
A retrospective analysis was conducted to record the patient’s disease history data, including gender, age, white blood cell (WBC), neutrophil, lymphocytes and high-sensitivity C-reactive protein (hs-CRP). AFU were measured using AFU Kit (Yanjin Biotechnology Co. Ltd., Shanghai, China). Briefly, after mixing plasma samples with ethylenediaminetetraacetic acid (EDTA) for 10–20 minutes, the samples were centrifuged for 20 minutes and the supernatants were collected. Next, according to the instructions, dilute the standard and add samples, seal the plate, and incubate at 37 collect for 30 minutes. Then shake off the liquid in the hole, fill each hole with washing solution, let it stand for 30 seconds, and then discard it. Repeat this process 5 times and pat dry. Add affinity chain enzyme horseradish peroxidase (HRP) to each well, incubate at 37 ℃ for 30 minutes, and wash. After that, add substrates A and B to each well, gently shake and mix well, and incubated in dark at 37 ℃ for 15 minutes. Finally, add 50 µL termination fluid to each hole and terminate the reaction. Measure the absorbance [optical density (OD) value] of each well in sequence at a wavelength of 450 nm.
Statistical analysis
Statistical analysis was performed using SPSS 24.0 (SPSS, Inc., Chicago, IL, USA). Measurement data were presented as the mean ± standard deviation [or median (Q1, Q3)] and analyzed by t-test or Mann-Whitney U test. Kolmogorov-Smirnov test was used for the detection of whether the data is normal distribution. Categorical data were presented as n and analyzed using χ2 test. The receiver operating characteristic (ROC) curve was used to determine the cut off value. P<0.05 was considered to indicate a statistically significant difference.
Results
The baseline data of individuals
A total of 190 individuals were included in this study containing 96 RA cases and 94 healthy controls, with the mean age of (59.62±13.44) years in RA group and (59.98±14.73) years in control group. As shown in Table 1, the results found significant differences in WBC (P<0.01), neutrophil (P<0.0001), lymphocytes (P<0.0001) and hs-CRP (P<0.0001) between the two groups. There were no statistically differences in age and gender (P>0.05).
The activity of AFU in peripheral blood of RA patients and healthy controls
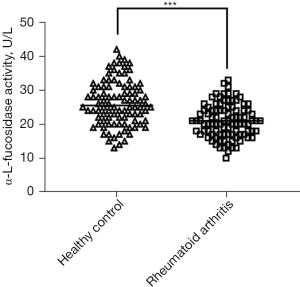
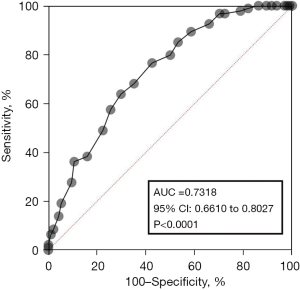
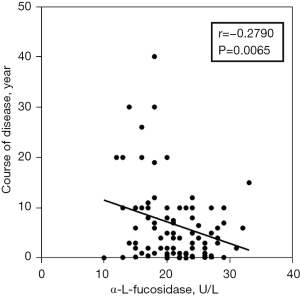
To compare the activity of AFU in peripheral blood of RA patients and healthy controls, the results indicated that AFU activity in peripheral blood of RA patients were lower than healthy controls (Figure 1). The ROC curves of specificity and sensitivity were shown in Figure 2. The area under the curve (AUC) was 0.7318, with the 95% confidence interval (CI) of 0.6610 to 0.8027, P<0.0001. Figure 3 shows a linear regression plot of correlation analysis between AFU activity and course of disease in RA patients. And the results demonstrated that the higher AFU activity, the shorter the course of disease (r=−0.2790, P=0.0065). Herein, the pooled results suggested that changes of peripheral blood AFU activity might be associated with progression of disease in RA patients.
Correlation between activity of AFU and glucose and lipid metabolism
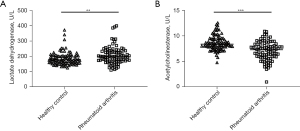
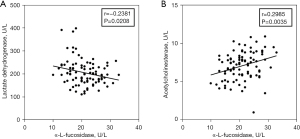
Finally, the differences of lactate dehydrogenase activity and acetylcholinesterase activity between RA and control groups were compared. As shown in Figure 4A,4B, the activity of lactate dehydrogenase in patients with RA is higher than that of healthy control, but the activity of acetylcholinesterase is lower than that of normal people. To further verify whether AFU activity is related to glucose and lipid metabolism, the correlation between AFU activity and the activity of lactate dehydrogenase (r=−0.2381, P=0.0208) and acetylcholinesterase (r=0.2985, P=0.0035) were analyzed (Figure 5A,5B). The overall results found that AFU activity was related to glucose and lipid metabolism, the changes of AFU activity may lead to disturbances in glucose and lipid metabolism.
Discussion
For all we know, this is the first study investigating an association between peripheral blood AFU activity changes and glucose and lipid metabolism. Herein, we proposed an integrated analysis of present evidences to investigate the potential association between the changes of peripheral blood AFU activity and glucose and lipid metabolism in RA patients. Totally 190 subjects were assessed including 96 RA patients and 94 controls. Our findings showed that peripheral blood AFU activity changes might be related to the disorders of glucose and lipid metabolism.
RA is a long-term and common chronic systemic inflammatory disease. Although the etiology of RA is still unclear, its relation to accelerated development of atherosclerosis and cardiovascular disease has been assessed (20,21). This may be attribute to the fact that obesity and dyslipidemia are risk factors for atherosclerosis and RA, while inflammation and immune abnormalities are the same pathogenic mechanisms of both diseases (22). There is also evidence of high prevalence of diabetes in patients with RA (23). Interleukin (IL)-1β, IL-6, and tumor necrosis factor are involved in RA, which can determine β cell dysfunction and destruction, gradually lead to insulin resistance and in turn to trigger diabetes (23,24). A cross-section study conducted by Ruscitti et al. (25) proposed a significantly correlation between RA and glucose metabolism abnormalities, as well as prolonged disease duration and joint damage, which may lead to metabolic syndrome.
AFU plays an important role in many biological behaviors, including immune responses, signal transduction and so on (26). Clinically, AFU is often served as a tumor marker in various cancers and its importance in the occurrence and progression of inflammatory and autoimmune diseases has been reviewed early (27). A research found that levels of AFU were significantly correlated to rheumatic diseases and the immune function of AFU may be a marker of rheumatism or chronic inflammatory manifestations (14). This research also pointed out that AFU activity in peripheral blood can be detected to further emphasize the relationship between glucose metabolism and immune response in RA patients. A more comprehensive study was performed to probe the role of AFU activity in glucose and lipid metabolism of RA. Our results found that the changes of AFU activity may lead to disturbances in glucose and lipid metabolism.
Endreffy et al. (13) conducted an investigation to further elucidated the function of AFU activity in rheumatic disorders. Research subjects included both pediatrics and adults, the results showed no correlation of AFU activity in the pediatric patients with metabolic syndrome. This may be due to the limitations of study subject selection. Besides, a relationship was found between plasma AFU activity and inflammatory disorders or metabolic syndrome in case and control group, which was consistent with our results. The current study found that disorders of glucose and lipid metabolism were observed in RA patients and peripheral blood AFU activity might be associated with progression of disease in RA patients.
Lactate dehydrogenase is an important enzyme system in glucose metabolism while acetylcholinesterase is an important indicator of lipid metabolism. For example, the present study firstly put forward a potential correlation between the changes of AFU activity and disorders glucose and lipid metabolism, in order to fully understand the specific mechanism of AFU in RA patients, which is benefit to reasonable application of AFU evaluation as a screen and monitoring tool (28).
In addition, several limitations should be mentioned. Firstly, neither the case nor the control represents the entire case or the population to which it belongs, geographical limitations always existed in the choice of subjects. Secondly, only 190 subjects were enrolled this research, which may affect the statistical power because of small sample sizes. In the future, large samples and more comprehensive studies are needed to verify our results and further explore the role of AFU activity in glucose and lipid metabolism of RA.
Conclusions
In summary, changes of peripheral blood AFU activity might be associated with progression of disease in RA patients. The changes of AFU activity may lead to disturbances in glucose and lipid metabolism.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-50/rc
Data Sharing Statement: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-50/dss
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-50/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-50/coif). X.G.Z. received fundings from the Scientific Research Project of Jiangsu Provincial Health Commission (No. Z2021088), and Nantong Science and Technology Bureau (No. JC2020019). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of The Second Affiliated Hospital of Nantong University (No. 083467820). All the patients voluntarily participated in the study and informed consent was obtained before enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023-38. [Crossref] [PubMed]
- McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017;389:2328-37. [Crossref] [PubMed]
- Liao KP. Cardiovascular disease in patients with rheumatoid arthritis. Trends Cardiovasc Med 2017;27:136-40. [Crossref] [PubMed]
- Xiao M, Fu X, Ni Y, et al. Protective effects of Paederia scandens extract on rheumatoid arthritis mouse model by modulating gut microbiota. J Ethnopharmacol 2018;226:97-104. [Crossref] [PubMed]
- Korani S, Korani M, Butler AE, et al. Genetics and rheumatoid arthritis susceptibility in Iran. J Cell Physiol 2019;234:5578-87. [Crossref] [PubMed]
- Li J, Che N, Xu L, et al. LC-MS-based serum metabolomics reveals a distinctive signature in patients with rheumatoid arthritis. Clin Rheumatol 2018;37:1493-502. [Crossref] [PubMed]
- Shaikh S, Dahani A, Arain SR, et al. Metabolic Syndrome In Young Rheumatoid Arthritis Patients. J Ayub Med Coll Abbottabad 2020;32:318-22. [PubMed]
- Kido A, Komatsu N, Oya M. alpha-L-fucosidase phenotyping in human placentae, semen and seminal stains. Forensic Sci Int 1986;30:37-43. [Crossref] [PubMed]
- Troost J, van der Heijden MC, Staal GE. Characterization of alpha-L-fucosidase from two different families with fucosidosis. Clin Chim Acta 1976;73:329-46. [Crossref] [PubMed]
- Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol 2006;12:1175-81. [Crossref] [PubMed]
- Stefaniuk P, Cianciara J, Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol 2010;16:418-24. [Crossref] [PubMed]
- Liu TW, Ho CW, Huang HH, et al. Role for alpha-L-fucosidase in the control of Helicobacter pylori-infected gastric cancer cells. Proc Natl Acad Sci U S A 2009;106:14581-6. [Crossref] [PubMed]
- Endreffy I, Bjørklund G, Szerafin L, et al. Plasma alpha-L-fucosidase activity in chronic inflammation and autoimmune disorders in a pediatric cohort of hospitalized patients. Immunol Res 2017;65:1025-30. [Crossref] [PubMed]
- Endreffy I, Bjørklund G, Bartha A, et al. Plasma α-L-fucosidase-1 in patients with Sjögren's syndrome and other rheumatic disorders. Int J Rheum Dis 2019;22:1762-7. [Crossref] [PubMed]
- Jiang M, Liu S, Jiang H, et al. Brain abnormalities in fucosidosis: transplantation or supportive therapy? Metab Brain Dis 2017;32:317-20. [Crossref] [PubMed]
- Arias-de la Rosa I, Escudero-Contreras A, Ruiz-Ponce M, et al. Pathogenic mechanisms involving the interplay between adipose tissue and auto-antibodies in rheumatoid arthritis. iScience 2022;25:104893. [Crossref] [PubMed]
- Ye L, Zhang X, Wu H, et al. Insulin resistance and adverse lipid profile in untreated very early rheumatoid arthritis patients: A single-center, cross-sectional study in China. Arch Rheumatol 2022;37:593-602. [Crossref] [PubMed]
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-24. [Crossref] [PubMed]
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569-81. [Crossref] [PubMed]
- Meune C, Touzé E, Trinquart L, et al. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2009;48:1309-13. [Crossref] [PubMed]
- Cavagna L, Boffini N, Cagnotto G, et al. Atherosclerosis and rheumatoid arthritis: more than a simple association. Mediators Inflamm 2012;2012:147354. [Crossref] [PubMed]
- Gonzalez A, Maradit Kremers H, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis 2008;67:64-9. [Crossref] [PubMed]
- Donath MY, Dinarello CA, Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat Rev Immunol 2019;19:734-46. [Crossref] [PubMed]
- Zuliani G, Morieri ML, Volpato S, et al. Insulin resistance and systemic inflammation, but not metabolic syndrome phenotype, predict 9 years mortality in older adults. Atherosclerosis 2014;235:538-45. [Crossref] [PubMed]
- Ruscitti P, Ursini F, Cipriani P, et al. Prevalence of type 2 diabetes and impaired fasting glucose in patients affected by rheumatoid arthritis: Results from a cross-sectional study. Medicine (Baltimore) 2017;96:e7896. [Crossref] [PubMed]
- Alhadeff JA, Janowsky AJ. Human serum alpha-L-fucosidase. Clin Chim Acta 1978;82:133-40. [Crossref] [PubMed]
- Ge W, Li D, Gao Y, et al. The Roles of Lysosomes in Inflammation and Autoimmune Diseases. Int Rev Immunol 2015;34:415-31. [Crossref] [PubMed]
- Malatt C, Koning JL, Naheedy J. Skeletal and Brain Abnormalities in Fucosidosis, a Rare Lysosomal Storage Disorder. J Radiol Case Rep 2015;9:30-8. [Crossref] [PubMed]
Cite this article as: Zhou XG, He H, Yuan K. Changes of peripheral blood α-L-fucosidase activity in patients with rheumatoid arthritis: a cross-sectional study. Ann Joint 2024;9:13.


