
The effect of nonsteroidal anti-inflammatory drug use on soft tissue and bone healing in the knee: a systematic review
Highlight box
Key findings
• Selective cyclooxygenase (COX)-2 inhibitors may impair soft tissue, bone, and tendon-to-bone healing in the knee of animals.
• Clinically, nonselective and selective COX-2 inhibitors do not impair anterior cruciate ligament and meniscus healing, although with limited evidence.
What is known and what is new?
• Selective COX-2 inhibitors are known to cause adverse effects in overall soft tissue, tendon-to-bone, and bone healing.
• In the knee, selective and nonselective COX-2 inhibitors may impair soft tissue, tendon-to-bone, and bone healing in animals but may not impair healing clinically.
What is the implication, and what should change now?
• Further clinical studies are needed to better understand the dose and duration dependent risks of nonsteroidal anti-inflammatory drugs in knee healing as current literature primarily consists of animal studies.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used to mitigate pain and inflammation associated with musculoskeletal conditions. Increased use of NSAIDs has been beneficial in diminishing postoperative opioid use; however, there remains conflicting data on the effect of NSAIDs on soft tissue and bone healing (1-7). NSAIDs function via inhibition of cyclooxygenase (COX), an enzyme responsible for prostaglandin synthesis. There are two isoforms of COX: COX-1 and COX-2. Several in vitro, animal, and clinical studies have identified COX-2 inhibitors to be the primary culprit of causing adverse effects in soft tissue, tendon-to-bone, and bone healing (2,4-6). In the knee, meniscal tissue and intraarticular ligament reconstruction already have limited healing capacity (1). Therefore, there is a need for identifying potential adverse effects in the knee from the perioperative and postoperative use of NSAIDs to avoid decreased or delayed healing and reconstruction graft failure.
The currently available systematic and scoping review articles have taken broader approaches in identifying the effect of NSAID use on tissue healing (2-5,8). However, there is limited information to identify the effect of NSAIDs on tissue healing specifically in the knee, where meniscal repairs, cartilage surgeries and intra-articular ligament reconstructions may be susceptible to delayed or a lack of healing. The purpose of this study was to investigate the effect of NSAIDs on the healing of knee soft tissue and bone. The authors hypothesized that NSAIDs may have a negative effect on soft tissue and tendon-to-bone healing in the knee. We present this article in accordance with the PRISMA reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-58/rc).
Methods
This systematic review has been registered to the Prospective Register of Systematic Reviews (PROSPERO ID: CRD42023407331).
Study eligibility
Inclusion criteria for studies included the following: clinical studies investigating tissue healing in the knee after peri- or postoperative NSAID use, animal and in vitro studies investigating tissue healing in the knee after NSAID use, published in a peer-reviewed journal, and full English text available.
Exclusion criteria included the following: clinical, animal, and in vitro studies not related to knee healing and NSAID use, case reports, reviews, meta-analyses, opinion articles, technique articles, and studies with unavailable English text.
Search strategy
A literature search was conducted across the following electronic databases: PubMed/MEDLINE, Excerpta Medical Database (Embase)/Ovid, and the Cochrane Central Register of Controlled Trials. We developed an inclusive search strategy and conducted a search in August 2022 with no date restrictions employed. The following search strategy was utilized: (((nonsteroidal anti-inflammatory drug OR NSAID) AND (knee OR meniscus OR ACL OR MCL OR patella OR ligament OR tendon OR muscle OR soft tissue)) AND (healing)). After all articles were collected from the searches, duplicate articles were removed. Title and abstract screening were performed independently by two authors (R.H.S. and J.D.) using the inclusion and exclusion criteria. In studies where both authors agreed on inclusion, full text was reviewed. In cases where consensus was not reached on inclusion of a study, a third author (N.I.K.) evaluated and determined whether the study in question was included.
Risk of bias assessment
All included randomized clinical studies were evaluated for bias using the Cochrane bias assessment tool. All included non-randomized clinical studies were evaluated for bias using the Methodological Index for Non-Randomized Studies (MINORS) scoring system. MINORS is a validated assessment tool for methodological quality of studies by scoring non-comparative (0–16) and comparative studies (0–24), where higher scores indicate lower levels of bias. All included animal studies were evaluated for bias using the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) risk of bias assessment tool. SYRCLE is a validated tool that uses 10 “yes or no” questions, where more “yes” answers indicate lower risk of bias. As there is no validated or consistently utilized risk of bias assessment tool for in vitro studies, bias could not be assessed in these studies.
Data extraction
Two authors (R.H.S. and J.D.) extracted and validated all collected data from each included study. For clinical and animal studies, the data points collected included the number of subjects, sex, mean age, mean follow-up, type of pathology, procedure or intervention performed (if any), type of NSAID administered, NSAID dosage, timing of drug use, outcome measures, control group, and study results. For in vitro studies, the tested specimen, type of NSAID tested, outcome measures, and study results were collected. The type of NSAID administered was categorized as either selective COX-1, selective COX-2, or nonselective COX inhibitors. Selective inhibitors have preferential blockade of either the respective COX-1 or COX-2 enzyme. Nonselective inhibitors act on both COX enzymes to a significant degree without preferential blockade of either enzyme.
Statistical analysis
All statistical analyses were performed using Excel (Microsoft Corp, Redmond, WA, USA). All extracted data were analyzed using descriptive statistics, including weighted mean values and frequencies, due to the heterogeneity of reported data. Weighted mean values were calculated based on the sample size reported in each included study.
Results
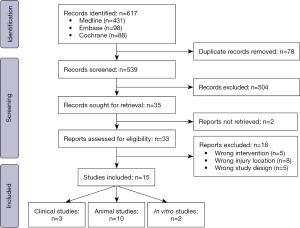
On initial search, a total of 617 articles were identified. After deduplication, 539 articles were screened based on title and abstract, of which 504 were excluded according to the inclusion and exclusion criteria. After full text analysis of 33 articles, studies were excluded due to the wrong intervention (n=5), injury location (n=8), or study design (n=5). We identified 15 articles for inclusion in our study (Figure 1).
Clinical studies
We identified three clinical studies assessing the use of NSAIDs on primarily anterior cruciate ligament (ACL) reconstructions and to a lesser extent on meniscal repairs across 8,009 patients (4,638 male, 3,371 female) (9-11). The weighted mean age of patients was 28.8 years with an average follow-up of 24.5 months (Table 1). The procedures studied included isolated ACL reconstruction (7,902/8,009) (9,11), isolated meniscus repair (64/8,009) (10), and combined meniscus repair and ACL reconstruction (43/8,009) (9-11).
Table 1
One study assessed selective COX-2 inhibitors (9), one study assessed a nonselective inhibitor (10), and one study assessed both selective COX-2 and nonselective COX inhibitors (11). None of the included studies found a significant increase in failure of each procedure with NSAID administration pre-, peri-, or post-operatively in comparison to placebo or no NSAID administration. Ge et al. found no significant difference in knee stability and subjective outcomes when comparing post-operative NSAID versus opiate administration after ACL reconstruction (9). The clinical study results are summarized in Table 2.
Table 2
| Study | Type of pathology | Intervention | NSAID administered [COX-selectivity]* | Dosage | Primary outcome measures | Secondary outcome measure(s) | Control group | Result |
|---|---|---|---|---|---|---|---|---|
| Ge et al. (9) | ACL tear (100%), meniscal tear (34%) | ACL reconstruction | Celecoxib [2] | 200 mg twice a day post-operatively | Knee stability | IKDC, Lysholm score, Tegner scale | Opioid | No significant difference |
| Proffen et al. (10) | Meniscal tear (100%), ACL tear (40%) | Meniscal repair +/–ACL reconstruction | Keterolac (non-selective) | 7.5–60 mg based on body weight peri-operatively | Reoperation, failure of meniscal repair | KOOS, SF-36 Health Survey, IKDC | No NSAID | No significant difference |
| Soreide et al. (11) | ACL tear (100%), meniscal tear (46%) | ACL reconstruction | Diclofenac [2], keterolac (non-selective), celecoxib [2], other | Not reported | Graft survival | KOOS-QOL score | No NSAID | No significant difference |
*, Selective inhibitors have preferential blockade of either the respective COX-1 (referred to as 1) or COX-2 (referred to as 2) enzyme. Nonselective inhibitors act on both COX enzymes to a significant degree without preferential blockade of either enzyme (referred to as non-selective). NSAIDs, nonsteroidal anti-inflammatory drugs; COX, cyclooxygenase; ACL, anterior cruciate ligament; IKDC, International Knee Documentation Committee Subjective Knee Form; KOOS-QOL, Knee Injury and Osteoarthritis Outcome Score-Quality of Life; SF-36, Short Form-36 Health Survey.
Animal studies
Ten animal studies investigated the effect of NSAIDS on knee healing across 785 animals (288 male, 497 female) (12-21). All animals included in each study were skeletally mature. The mean follow-up time ranged from 0.5–6 months. General study characteristics are summarized in Table 3. The primary knee injuries studied were medial collateral ligament (MCL) transections (504/785). Eight studies investigated soft tissue healing and two studies assessed bone healing. Seven studies did not use any repair intervention and allowed for MCL healing via scarring (12-14,16,17,20,21). Six studies assessed COX-2 inhibitors (14-16,18-20), four studies assessed COX-1 inhibitors (12,13,15,16), and four studies assessed non-selective COX inhibitors (Table 4) (15-17,21).
Table 3
| Study | Number of animals (male/female) | Strain (failure load)† | Animal type (body weight)* | Dosage (mg/kg) | Length of follow-up |
|---|---|---|---|---|---|
| Bogatov et al. (12) | 80 (M) | 21–55 N/kg | Rats (592 g) | Low dose: 10.6 | 2 weeks |
| High dose: 28.1 | |||||
| Dahners et al. (13) | 140 (M) | 28–52 N/kg | Rats (450 g) | 5 | 2 weeks |
| Elder et al. (14) | 50 (M) | 21–53 N/kg | Rats (515 g) | 5–30 | 2 weeks |
| Ferry et al. (15) | 215 (F) | 52–72 N | Rats (425 g) | 2.5–60 | 2 weeks |
| Hanson et al. (16) | 150 (F) | 37–48 N/kg | Rats (430 g) | 2.5–60 | 2 weeks |
| Moorman et al. (17) | 24 (F) | 24–115 N | Rabbits (NR) | 70 | 4 weeks |
| Sauerschnig et al. (18) | 32 (F) | 28–69 N | Rabbits (3.5 kg) | 10 | 3 weeks |
| Taroni et al. (19) | 16 (F) | NA | Dogs (40 kg) | NR | 6 months |
| Warden et al. (20) | 60 (F) | 13–35 N | Rats (275 g) | 5 | 12 weeks |
| Watson et al. (21) | 18 (M) | NA | Rabbits (4.25 kg) | 2.1–3 | 70 days |
†, strain was applied at a constant rate of 0.25 mm/s for all studies that assessed load to failure and is expressed as a range. *, Body weight is expressed as an average value. NSAID, nonsteroidal anti-inflammatory drug; M, male; F, female; NR, not reported; NA, not assessed.
Table 4
| Study | Type of pathology | Intervention | NSAID administered [COX-selectivity] | Primary outcome measure | Control group | Result |
|---|---|---|---|---|---|---|
| Bogatov et al. (12) | MCL transection | Allowed to heal via scarring | SC-560 [1] | Failure load | Placebo | Unaffected strength of healing ligament |
| Increased strength of contralateral ligament | ||||||
| Dahners et al. (13) | MCL transection | Allowed to heal via scarring | Piroxicam [1] | Failure load | Placebo | Increased early strength of healing ligament but final strength unaffected |
| Unaffected strength of uninjured ligaments | ||||||
| Elder et al. (14) | MCL transection | Allowed to heal via scarring | Celecoxib [2] | Failure load | Placebo | Decreased strength of healing ligament |
| Unaffected strength of uninjured ligaments | ||||||
| Ferry et al. (15) | Patellar tendon transection | Stabilization of patella with cerclage suture | Ibuprofen [non-selective], naproxen [non-selective], piroxicam [1], celecoxib [2], valdecoxib [2] | Failure load | Placebo | Unaffected strength of healing tendon for ibuprofen and naproxen but decreased with piroxicam, celecoxib, and valdecoxib |
| Unaffected strength of uninjured limbs | ||||||
| Hanson et al. (16) | MCL transection | Allowed to heal via scarring | Naproxen [non-selective], piroxicam [1], rofecoxib [2] | Failure load | Placebo | Increased strength of healing ligament for piroxicam and unaffected with naproxen and rofecoxib |
| Unaffected strength of uninjured limbs | ||||||
| Moorman et al. (17) | MCL transection | Allowed to heal via scarring | Ibuprofen [non-selective] | Failure load | Placebo | Unaffected strength of healing tendon |
| Unaffected strength of uninjured ligaments | ||||||
| Sauerschnig et al. (18) | ACL resection | ACL reconstruction | Celecoxib [2] | PGE2 in synovium; bone formation | Placebo | Decreased tendon-to-bone healing |
| Taroni et al. (19) | Cranial cruciate ligament rupture | Tibial plateau osteotomy | Firocoxib [2] | Bone healing | Intra-articular MSCs | Decreased early healing but final clinical scores unaffected |
| Warden et al. (20) | MCL transection | Allowed to heal via scarring | Celecoxib [2] | Failure load | Placebo | Decreased early healing but final strength unaffected |
| Watson et al. (21) | Knee arthrosis | Allowed to heal via scarring | Indomethacin [non-selective] | Microscopic findings | No NSAID | Unaffected healing of cartilage but fewer granules over chondrocytes |
NSAIDs, nonsteroidal anti-inflammatory drugs; COX, cyclooxygenase; MCL, medial collateral ligament; ACL, anterior cruciate ligament; PGE2, prostaglandin E2; MSCs, mesenchymal stromal cells.
Among studies assessing COX-2 inhibitor effects on soft tissue, healing was impaired (2/4) (14,15), delayed but unaffected (1/4) (20), or unaffected (1/4) (16). For bone healing, Taroni et al. noted delayed but unaffected tibial osteotomy healing after administration of a COX-2 inhibitor, while Sauerschnig et al. noted a decrease in tendon-to-bone healing in ACL reconstruction tunnels (18,19). In studies assessing COX-1 inhibitors, ligament healing after transection was either increased (1/4) (16), unaffected (2/4) (12,13), or impaired (1/4) (15). After administration of nonselective COX inhibitors, soft tissue (3/3) (15-17) and cartilage (1/1) (21) healing were unaffected in all studies.
In vitro studies
Two in vitro studies investigating the effect of NSAIDs on knee specimens through cell testing were identified (22,23). Both studies identified a negative outcome on cell proliferation or viability after nonselective COX inhibition. Riley et al. identified no negative outcome on patellar tendon cell proliferation from selective COX-2 inhibition (22); however, Schwarting et al. found reduced cell viability among ACL 3T3 cell lines (23). The in vitro study results are summarized in Table 5.
Table 5
| Study | Specimen | NSAID tested [COX-selectivity] | Dosage | Primary outcome measure | Result |
|---|---|---|---|---|---|
| Riley et al. (22) | Patellar tendon | Indomethacin [non-selective], naproxen [non-selective], diclofenac [2], aceclofenac [2] | Indomethacin: 20 µg/mL | Tendon cell proliferation | Indomethacin and naproxen inhibited cell proliferation, while diclofenac and aceclofenac had no significant effect |
| Naproxen: 100 µg/mL | |||||
| Diclofenac: 2 µg/mL | |||||
| Aceclofenac: 10 µg/mL | |||||
| Schwarting et al. (23) | ACL MC3T3 and 3T3 cell lines | Ibuprofen [non-selective], parecoxib [2] | Ibuprofen: 5–100 µM | Cell viability | Both ibuprofen and parecoxib reduced cell viability |
| Parecoxib: 5–100 µM |
NSAIDs, nonsteroidal anti-inflammatory drugs; COX, cyclooxygenase; ACL, anterior cruciate ligament.
Risk of bias assessment
Three non-randomized comparative clinical studies were assessed using the MINORS scoring system and were determined to have a low risk of bias (Table S1) (9-11). The ten included animal studies were evaluated using the SYRCLE risk of bias assessment tool (12-21). Nine of these animal studies were determined to have a low risk of bias and one was determined to have an unclear risk of bias (Table S2).
Discussion
The most important finding of the present study was that nonselective and selective COX-2 inhibitor administration among patients who underwent ACL reconstruction and meniscus repair did not affect postoperative stability, subjective outcomes, and either ACL graft failure or meniscal healing. Among animal studies on postoperative NSAID use after knee surgery, it was reported that administration of selective and nonselective COX-2 inhibitors may impair healing of extra-articular soft tissue, bone and tendon-to-bone.
Limited clinical studies currently exist in assessing the effects of NSAIDs on knee, soft tissue, and bone healing. While only three clinical studies were identified in our systematic review, these studies identified no significant differences in postoperative stability, subjective outcomes, and either ACL graft failure or meniscal healing between the NSAID recipients and control groups (9-11). Similar clinical studies assessing NSAID use after rotator cuff repair have caused concern specifically for selective COX-2 inhibitor use. Oh et al. reported postoperative use of selective COX-2 inhibitors increased the rate of rotator cuff retear compared to nonselective COX inhibitors (24). However, the failure rate was relatively higher for large rotator cuff repairs and should not be directly compared with the different physiologic environment of the knee (25-27). This difference could perhaps be attributed to the difference in environment between the ACL (intra-articular) and the rotator cuff insertions (mostly extra-articular/subacromial).
The animal studies included in this systematic review had greater variability in healing outcomes compared to the clinical studies. Nonselective COX inhibitors were found to not affect soft tissue and cartilage healing across studies (15-17,21). Selective COX-1 inhibitors were found to either improve or not affect ligament healing in three studies assessing MCL transection models, while one study by Ferry et al. found a negative effect on patellar tendon healing (12,13,15,21). Four articles found that soft tissue healing after selective COX-2 inhibitor use was either impaired, delayed, or unaffected (14-16,20). It is worth noting that male rats were used in the study by Elder et al. to investigate healing after selective COX-2 inhibitor use (14). Male rats are known to have a quick metabolism for two COX-2 inhibitors in particular: rofecoxib and celecoxib (28,29). The quick metabolism of male rats for COX-2 inhibitors may apply to other NSAIDs, making the use of female rats preferable. The half-life of COX-2 inhibitors is more comparable between female rats and humans (29,30). However, the medication dosages given to animals must be adequately adjusted to assess impaired healing, because small animals tend to have a higher metabolism than humans. In a study on the effects of perioperative parecoxib use on tibial shaft fracture healing, Hjorthaug et al. utilized allometric scaling based on calorific demand to determine parecoxib dosages in rats and allow for an adequate comparison to humans; an increase in dosage by a factor of four was required in the rat models (31).
In regard to healing of bone and intra-articular structures, dose duration and location of the structure of interest seems to be of great importance. Sauerschnig et al. reported impaired tendon-to-bone healing after ACL reconstruction in rabbits, suggesting that ACL graft healing in reconstruction tunnels may be compromised or delayed with selective COX-2 inhibitor use (18). However, these conclusions are not wholly defended by their data. They were able to demonstrate notable intra-articular prostaglandin E2 (PGE2) differences amongst control and COX-2 cohorts, however, they were unable to demonstrate any notable difference between intra-articular characteristics of the soft tissue portion of the grafts at 3 or 6 weeks between control groups and COX-2 administered groups. Further, the changes in bone density and new bone formation seem to be attributed to PGE2 levels. However, they found PGE2 levels rebounded to normal or elevated levels after cessation of COX-2 inhibitor administration, thereby suggesting that bone healing is restored when treatment is concluded. This is consistent with prior literature by Taroni et al. who found COX-2 inhibitor administration can lead to delayed but ultimately unimpaired bony healing in tibial osteotomies (19).
Another interesting finding by Saurschnig et al. was that delayed bony healing seemed to be different based upon location, with less bone formation in the midsection of the tunnel when compared to the aperture, meaning the bone nearest to the intra-articular space was less impaired (18). This is similar to prior literature which has shown the negative effect of COX inhibitors on bone healing, primarily in diaphyseal fractures with no effect in the metaphysis (32,33). Also furthering the argument of treatment duration being an additional variable of importance, an animal study on diaphyseal tibia fracture healing in rats demonstrated no negative effect of immediate or delayed short-term administration of parecoxib, a selective COX-2 inhibitor (31). These findings may be applicable to the clinical setting, regarding both the healing of diaphyseal fractures and soft tissue knee structures.
Both in vitro studies on the effect of nonselective and selective COX-2 inhibitors on patellar tendon and ACL cell lines identified decreased cell proliferation and viability (22,23). It is unclear if the reduced cell proliferation and viability identified in these studies would translate to delayed or impaired healing in a clinical model. Nonetheless, these studies provide insight into potential mechanisms for delayed and impaired healing caused by COX inhibitors that were identified in animal studies.
There were some limitations to our study. The number of clinical studies identified in our search were limited, with animal studies comprising the majority of included articles. While all the clinical studies assessed administration of NSAIDs in patients who underwent ACL reconstruction and/or meniscus repair, the animal studies assessed healing of the knee after transection of the MCL, ACL reconstruction, or patellar tendon repair. The transection model in animals makes for a different physiologic environment for healing than the clinical studies assessing surgical reconstruction. Additionally, medication dosage, duration, and average study follow-up time varied across studies. Lastly, a meta-analysis was not possible due to the heterogeneous presentation of the data.
Conclusions
Animal studies on postoperative NSAID use after knee surgery suggest that administration of selective and nonselective COX-2 inhibitors may impair healing; however, clinical studies demonstrated no detrimental effects on meniscal sutures and ACL reconstructions. Further clinical studies are needed to better characterize dose and duration dependent risks.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Inflammation of the Tibiofemoral Joint: Inflammatory Mediators, Treatment, and Long-Term Effects”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-58/rc
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-58/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-58/coif). The series “Inflammation of the Tibiofemoral Joint: Inflammatory Mediators, Treatment, and Long-Term Effects” was commissioned by the editorial office without any funding or sponsorship. N.N.D. served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Joint from August 2022 to July 2024. N.I.K. receives the Consulting fees from Vericel. R.F.L. is a consultant for Ossur, Smith & Nephew, and Responsive Arthroscopy; collects royalties from Ossur, Smith & Nephew, Elsevier, and Arthrex; has research grants from Ossur, Smith & Nephew, AANA, AOSSM; is on the committees for ISAKOS, AOSSM, AANA; and is on the editorial boards for AJSM, JEO, KSSTA, JKS, JISPT, OTSM. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Trasolini NA, Yanke AB, Verma NN, et al. Safety and Efficacy of Postoperative Nonsteroidal Anti-inflammatory Drugs in Sports Medicine. J Am Acad Orthop Surg 2022;30:535-42. [Crossref] [PubMed]
- Constantinescu DS, Campbell MP, Moatshe G, et al. Effects of Perioperative Nonsteroidal Anti-inflammatory Drug Administration on Soft Tissue Healing: A Systematic Review of Clinical Outcomes After Sports Medicine Orthopaedic Surgery Procedures. Orthop J Sports Med 2019;7:2325967119838873. [Crossref] [PubMed]
- Duchman KR, Lemmex DB, Patel SH, et al. The Effect of Non-Steroidal Anti-Inflammatory Drugs on Tendon-to-Bone Healing: A Systematic Review with Subgroup Meta-Analysis. Iowa Orthop J 2019;39:107-19. [PubMed]
- Ghosh N, Kolade OO, Shontz E, et al. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Their Effect on Musculoskeletal Soft-Tissue Healing: A Scoping Review. JBJS Rev 2019;7:e4. [Crossref] [PubMed]
- Chen MR, Dragoo JL. The effect of nonsteroidal anti-inflammatory drugs on tissue healing. Knee Surg Sports Traumatol Arthrosc 2013;21:540-9. [Crossref] [PubMed]
- Dimmen S. Effects of Cox inhibitors on bone and tendon healing. Acta Orthop Suppl 2011;82:1-22. [Crossref] [PubMed]
- Borgeat A, Ofner C, Saporito A, et al. The effect of nonsteroidal anti-inflammatory drugs on bone healing in humans: A qualitative, systematic review. J Clin Anesth 2018;49:92-100. [Crossref] [PubMed]
- Wheatley BM, Nappo KE, Christensen DL, et al. Effect of NSAIDs on Bone Healing Rates: A Meta-analysis. J Am Acad Orthop Surg 2019;27:e330-6. [Crossref] [PubMed]
- Ge H, Liu C, Shrestha A, et al. Do Nonsteroidal Anti-Inflammatory Drugs Affect Tissue Healing After Arthroscopic Anterior Cruciate Ligament Reconstruction? Med Sci Monit 2018;24:6038-43. [Crossref] [PubMed]
- Proffen BL, Nielson JH, Zurakowski D, et al. The Effect of Perioperative Ketorolac on the Clinical Failure Rate of Meniscal Repair. Orthop J Sports Med 2014;2:2325967114529537. [Crossref] [PubMed]
- Soreide E, Granan LP, Hjorthaug GA, et al. The Effect of Limited Perioperative Nonsteroidal Anti-inflammatory Drugs on Patients Undergoing Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2016;44:3111-8. [Crossref] [PubMed]
- Bogatov VB, Weinhold P, Dahners LE. The influence of a cyclooxygenase-1 inhibitor on injured and uninjured ligaments in the rat. Am J Sports Med 2003;31:574-6. [Crossref] [PubMed]
- Dahners LE, Gilbert JA, Lester GE, et al. The effect of a nonsteroidal antiinflammatory drug on the healing of ligaments. Am J Sports Med 1988;16:641-6. [Crossref] [PubMed]
- Elder CL, Dahners LE, Weinhold PS. A cyclooxygenase-2 inhibitor impairs ligament healing in the rat. Am J Sports Med 2001;29:801-5. [Crossref] [PubMed]
- Ferry ST, Dahners LE, Afshari HM, et al. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med 2007;35:1326-33. [Crossref] [PubMed]
- Hanson CA, Weinhold PS, Afshari HM, et al. The effect of analgesic agents on the healing rat medial collateral ligament. Am J Sports Med 2005;33:674-9. [Crossref] [PubMed]
- Moorman CT 3rd, Kukreti U, Fenton DC, et al. The early effect of ibuprofen on the mechanical properties of healing medial collateral ligament. Am J Sports Med 1999;27:738-41. [Crossref] [PubMed]
- Sauerschnig M, Stolberg-Stolberg J, Schmidt C, et al. Effect of COX-2 inhibition on tendon-to-bone healing and PGE2 concentration after anterior cruciate ligament reconstruction. Eur J Med Res 2018;23:1. [Crossref] [PubMed]
- Taroni M, Cabon Q, Fèbre M, et al. Evaluation of the Effect of a Single Intra-articular Injection of Allogeneic Neonatal Mesenchymal Stromal Cells Compared to Oral Non-Steroidal Anti-inflammatory Treatment on the Postoperative Musculoskeletal Status and Gait of Dogs over a 6-Month Period after Tibial Plateau Leveling Osteotomy: A Pilot Study. Front Vet Sci 2017;4:83. [Crossref] [PubMed]
- Warden SJ, Avin KG, Beck EM, et al. Low-intensity pulsed ultrasound accelerates and a nonsteroidal anti-inflammatory drug delays knee ligament healing. Am J Sports Med 2006;34:1094-102. [Crossref] [PubMed]
- Watson M. The suppressing effect of indomethacin on articular cartilage. Rheumatol Rehabil 1976;15:26-30. [Crossref] [PubMed]
- Riley GP, Cox M, Harrall RL, et al. Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by non-steroidal anti-inflammatory drugs in vitro. J Hand Surg Br 2001;26:224-8. [Crossref] [PubMed]
- Schwarting T, Pretzsch S, Debus F, et al. The Effect of Cyclooxygenase Inhibition on Tendon-Bone Healing in an In Vitro Coculture Model. Mediators Inflamm 2015;2015:926369. [Crossref] [PubMed]
- Oh JH, Seo HJ, Lee YH, et al. Do Selective COX-2 Inhibitors Affect Pain Control and Healing After Arthroscopic Rotator Cuff Repair? A Preliminary Study. Am J Sports Med 2018;46:679-86. [Crossref] [PubMed]
- Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 2004;86:219-24. [Crossref] [PubMed]
- Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg 2012;21:1753-63. [Crossref] [PubMed]
- Toussaint B, Schnaser E, Bosley J, et al. Early structural and functional outcomes for arthroscopic double-row transosseous-equivalent rotator cuff repair. Am J Sports Med 2011;39:1217-25. [Crossref] [PubMed]
- Halpin RA, Geer LA, Zhang KE, et al. The absorption, distribution, metabolism and excretion of rofecoxib, a potent and selective cyclooxygenase-2 inhibitor, in rats and dogs. Drug Metab Dispos 2000;28:1244-54. [PubMed]
- Paulson SK, Zhang JY, Breau AP, et al. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug Metab Dispos 2000;28:514-21. [PubMed]
- Paulson SK, Hribar JD, Liu NW, et al. Metabolism and excretion of [(14)C]celecoxib in healthy male volunteers. Drug Metab Dispos 2000;28:308-14. [PubMed]
- Hjorthaug GA, Søreide E, Nordsletten L, et al. Short-term perioperative parecoxib is not detrimental to shaft fracture healing in a rat model. Bone Joint Res 2019;8:472-80. [Crossref] [PubMed]
- Sandberg O, Aspenberg P. Different effects of indomethacin on healing of shaft and metaphyseal fractures. Acta Orthop 2015;86:243-7. [Crossref] [PubMed]
- Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br 2003;85:700-5. [Crossref] [PubMed]
Cite this article as: Solaiman RH, Dirnberger J, Kennedy NI, DePhillipo NN, Tagliero AJ, Malinowski K, Dimmen S, LaPrade RF. The effect of nonsteroidal anti-inflammatory drug use on soft tissue and bone healing in the knee: a systematic review. Ann Joint 2024;9:3.


