Glenoid bone loss in shoulder arthroplasty: a narrative review
Introduction
Background
Since the development of the modern reverse total shoulder replacement reverse shoulder arthroplasty (RSA), there has been an exponential increase in its utilization, with expanding indications including the management of severe rotator cuff arthropathy, massive rotator cuff tears with pseudo paralysis, arthritis, and proximal humeral fractures. Anatomic total shoulder arthroplasty (TSA) continues to remain the preferred surgical option for addressing primary osteoarthritis of the glenohumeral joint with intact cuff presenting with pain and disability (1-6).
Shoulder arthroplasty can effectively treat several pathologies and provide long-lasting improvement in pain and function. Shoulder replacement in the setting of osteoarthritis is a highly successful procedure with significant improvement for patients with respect to pain, function, and satisfaction (7).
When considering shoulder arthroplasty, the treating surgeon will often encounter significant glenoid defects, with reported prevalence rates as high as 39% (8). Glenoid deficiency can present in various forms, including superior, often seen in cases of rotator cuff tear arthropathy (CTA), posterior, commonly seen in cases of primary osteoarthritis or glenoid dysplasia, and anterior, as often seen in patients with chronic anterior instability arthropathy. Significant medial erosion may be observed in patients with primary osteoarthritis or inflammatory arthritis, while more extensive defects may be found in revision cases (8). Thus, the treating surgeon must be able to identify and address such defects appropriately.
Rationale and knowledge gap
Failure to assess and appropriately manage glenoid bone loss (GBL) can result in poor initial fixation of the glenoid component whether the surgeon is implanting an anatomic or reverse total shoulder replacement. Poor fixation of the glenoid compound at time zero can result in an increased risk of early implant loosening, suboptimal clinical outcomes, and reduced implant longevity (9-14). In fact, Farron et al. used a finite element analysis model to evaluate torque at the cement-bone interface with increasing degrees of glenoid retroversion (10). They found that, with over 10 degrees of implanted glenoid retroversion, an increase of over 700% in micro-motion at the cement-bone interface was identified along with a 326% increase in contact stresses (10). This is a critical factor, given that up to 15% of patients with glenohumeral arthritis have some degree of posterior GBL, making the implantation of the glenoid prosthesis in the optimal position challenging if the bony defect is not addressed appropriately (10). The presence of significant GBL is increased in revision shoulder arthroplasty when compared to primary arthroplasty, and inadequate glenoid bone stock can negatively impact the outcomes of RSA (7). To minimize the risk of early implant loosening and failure, careful preoperative planning with the goal of maintaining an appropriate glenoid version and ensuring proper implant positioning and robust fixation are crucial (7,11).
Objective
The aim of this review is to address the importance of evaluating and managing GBL in shoulder arthroplasty. The article provides an overview of the topic, discusses relevant classifications, and emphasizes the need for individualized treatment based on the best available evidence. Moreover, various approaches have been proposed to deal with GBL, which require a tailored assessment of the defect to provide optimal fixation. The article aims to provide guidance based on the recent literature on GBL in shoulder arthroplasty. We present this article in accordance with the Narrative Review reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-24/rc).
Methods
PubMed, MEDLINE, EMBASE, AccessMedicine, ClinicalKey, DynaMed, and Micromedex were queried for publications utilizing the following keywords: “glenoid bone loss” AND “glenoid bone deficiency” AND “shoulder arthroplasty” AND “classification”. The search was restricted to research published over the last 20 years. MeSH and EMTREE terms were utilized in various combinations to increase search sensitivity. We focused attention on recently published research with effort taken to include the highest level of available evidence where possible. See Table 1 for details pertaining to the search strategy.
Table 1
| Items | Specification |
|---|---|
| Date of search | Feb 23 2023 |
| Databases and other sources searched | PubMed, MEDLINE, EMBASE, AccessMedicine, ClinicalKey, DynaMed, and Micromedex |
| Search terms used | “glenoid bone loss” AND “glenoid bone deficiency” AND “shoulder arthroplasty” AND “classification” |
| Timeframe | 2004 to 2023 |
| Inclusion criteria | All studies related to total shoulder arthroplasty and the management of glenoid bone loss. No restriction for study type or language |
| Selection process | Search conducted by single author and results of the search were screened by two authors (A.A., S.A.) for relevance |
Findings and discussion
Etiologies of GBL
Correction of GBL often requires adjustments in multiple planes, as the pattern of bone loss tends to correspond to the underlying condition. Patients with CTA generally present with superior glenoid bone erosion, along with posterior erosion for those with primary arthritis, and anterior erosion for those with chronic shoulder instability. In addition, inflammatory arthritis tends to have central erosion. Revision shoulder arthroplasty has no specific GBL pattern (2).
Primary glenohumeral osteoarthritis
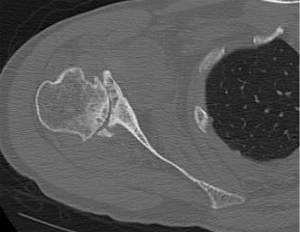
More than 50% of individuals with glenohumeral osteoarthritis have abnormal glenoid morphology and/or humeral head subluxation, with the most common being posterior humeral head subluxation, often presenting with posterior GBL (refer to Figure 1) (1-3,13-18).
CTA
Patients with CTA frequently exhibit abnormal glenoid morphology and bone loss, with a reported incidence of almost 40% (18). Unlike osteoarthritis, where GBL mainly occurs in the sagittal plane, patients with CTA typically have bone loss in the axial plane, primarily posterior-superiorly. This is primarily attributed to the mechanical forces and imbalances created by the chronic rotator cuff tears, which in turn fail to provide adequate support to the humeral head. As a result, the humeral head may migrate superiorly, creating abnormal contact and impingement between the humeral head and the acromion—eventually leading to erosion of the superior aspect of the humeral head (1-3,13,18).
Inflammatory arthritis
In patients with inflammatory arthritis, the most common pattern of GBL involves the central region, leading to significant medialization of the glenohumeral joint. Additionally, a significant proportion of these patients also have concomitant rotator cuff pathology. Central glenoid wear is problematic as patients may have an intact rotator cuff but have severe functional limitations due to the mechanical disadvantage of a medialized glenohumeral joint (1-3,18).
Generally, when managing any glenoid defect, the goal is to restore appropriate joint line. It is also important to re-establish the scapular neck length and lateralize the glenoid when significant medial erosion is present to avoid inferior glenoid notching and optimize the tensioning and lever arms of the deltoid muscle as well as the resting length of the residual rotator cuff.
Revision arthroplasty
During revision shoulder arthroplasty, GBL is a common issue that may result in difficulty with obtaining optimal baseplate fixation leading to the early loosening of glenoid components if left unaddressed. In contrast to other scenarios such as osteoarthritis, inflammatory arthritis, and CTA, there is no typical pattern of GBL in revision shoulder arthroplasty. As such careful preoperative planning is essential for the treating surgeon to appropriately plan options for bone loss management. The extent of bone loss can vary greatly depending on the previous implant and fixation, mode of failure, and can change substantially during the removal process (2,3,13,18).
Classification of GBL
Various classification systems have been developed over the years to categorize important aspects of GBL and guide treatment decisions. Classification based on glenoid morphology has been useful in identifying challenges related to bone and soft tissue deformities. These wear patterns have been shown to have a negative impact on the outcomes of unconstrained arthroplasty. Various classification systems exist based on the direction of glenoid erosion and containment status. Knowledge of available classification systems can be helpful when considering available treatment options.
Based on axial plane glenoid morphology
Walch et al. described a classification system based on axial plane glenoid morphology. This classification divided the pathology into three main categories, which was later modified to four categories by Bercik et al. to include subtypes. The classification is as follows: Type A: inflammatory osteoarthritis (further divided into A1 and A2), Type B: primary osteoarthritis (further divided into B1 and B2), Type C: dysplasia, and Type D: chronic anterior instability (19). Types B2 and C present the most severe cases of GBL. A B2-type glenoid is characterized by glenoid biconcavity consisting of both a paleo glenoid (native glenoid) as well as a neo glenoid (new glenoid) and often have varying degrees of posterior humeral head subluxation, while a C-type glenoid is characterized by retroversion greater than 25° (1-3,7,11,12,17,19-21).
Rispoli et al. proposed a grading system based on the degree of medialization of the glenohumeral joint in the coronal plane, which is directly related to the degree of subchondral plate erosion (22). If the subchondral plate is fully visible, then no erosion is considered present. If part or all of the plate is eroded, but to a depth of less than 5 mm, the glenoid is considered to have only mild erosion. When the erosion approaches the lateral aspect of the base of the coracoid process, typically resulting in 5 to 10 mm of erosion, it is considered moderate and finally erosion beyond the lateral aspect of the base of the coracoid, typically a depth of more than 10 mm, is considered severe glenoid erosion (17,22).
The Levigne classification system is designed to classify the different patterns of central GBL that occur in patients with rheumatoid arthritis. This system comprises three stages: Stage 1, which features minor central erosion; Stage 2, which involves central erosion extending to the level of the coracoid; and finally Stage 3, which involves central erosion extending medial to the level of the coracoid. The classification is particularly useful in cases of significant glenoid medialization, as it can help surgeons identify the critical need to restore and lateralize the glenoid which will result in significant improvements with respect to shoulder function and stability, and decrease the risk of subsequent scapular notching (2,3,16).
Based on coronal plane glenoid morphology
The Sirveaux Favard classification can be used to classify the location and severity of glenoid wear related to CTA in the vertical plane. This classification system outlines patterns of superior GBL frequently observed in CTA. It considers E0 involving superior humeral head migration with no glenoid erosion, E1 which involves concentric central erosion of the glenoid, E2 which involves superior erosion of the glenoid, E3 which involves superior erosion of the glenoid that extends inferiorly, and E4 which involves inferior erosion of the glenoid. This classification system is especially helpful for planning how to compensate for superior wear and to avoid the error of placing the baseplate in a superiorly tilted position if not addressed appropriately (2,3,12,17,23,24).
The classification system proposed by Habermeyer et al. is based on the relationship of a line drawn from the superior to the inferior glenoid rim in comparison to a vertical line at the level of the coracoid. It can be used to determine the degree of inferior tilt and erosion of the glenoid, which provides valuable information on inferior and superior bone loss which may not be fully appreciated when utilizing the classification system of Walch (23,25).
Combined axial and coronal plane glenoid morphology/CTA
Various radiographic classifications have been introduced for rotator cuff deficient shoulders, with the classification system by Neer et al. being one of the first to describe the morphologic changes associated with rotator cuff deficiency (26). Hamada et al. described five stages of radiographic changes for CTA, which define the degenerative changes that occur in the shoulder joint due to a massive rotator cuff tear (27). Visotsky et al. described the radiographic wear pattern for arthropathy caused by a significant rotator cuff tear, which is based on the displacement of the humeral head towards the medial or proximal direction due to the advancement of the disease. The authors highlight the importance of joint stability in determining the appropriate treatment approach (28).
Contained and uncontained defects
Classifying bone loss in revision cases can be more challenging. Antuna et al. developed a system for categorizing glenoid bony deficiency intraoperatively during revision surgery, based on the location and extent of erosion (29). This system includes three classifications: central, peripheral, and combined, with each classification further divided into mild, moderate, or severe (1-3,11,23).
The system proposed by Antuna et al. for glenoid bony deficiency in revision situations was later modified by Seidl and Williams to be used in both primary and revision settings (2,29). Furthermore, Page et al. developed a modification of the Antuna classification system to assist with impaction grafting during revision surgeries (30). The modified system classifies defects into three types: Type 1, which are contained and can be treated with impaction grafting; Type 2, which are uncontained but can be converted to Type 1 by utilizing a mesh or cortical graft; and Type 3, which are uncontainable and not amenable to impaction grafting (30).
Antuna et al. later proposed a modified classification system building on the work of Page et al. to describe all patterns of glenoid wear (29,30). This system categorizes defects as either centric (C1–4, based on the degree of vault destruction) or eccentric (E1–4, based on the percentage of the defect and its location, such as anterior or posterior). Within the eccentric category, defects are further subdivided based on the percentage of the defect, with E1 representing a minimal defect (<30%), E2 representing a moderate defect (30–60%), and E3 representing a severe defect (>60%) (23,31).
Frankle et al. developed a classification system for glenoid morphology based on three-dimensional computed tomography (3D-CT) models, which classifies glenoid pathology based on the quadrant of bone loss (posterior, superior, global, or anterior) (3,17,32). The study analyzed 216 glenoids and found that 3D-CT models significantly improved the reliability of identifying and characterizing deformities compared to previous two-dimensional CT and plain radiograph-based classification systems. This allowed for better determination of bone availability for baseplate and peripheral screw purchase. In cases of significantly altered glenoid morphology, standard implant techniques may not provide sufficient bone stock to support a glenoid baseplate, requiring altered techniques for stable and reliable fixation (32). Failure to recognize and account for bone loss may result in malpositioning of the glenosphere, leading to increased shear forces and component failure (17).
In summary, a comprehensive understanding of the glenoid bony defect is crucial for both primary and revision surgery and is a key factor in the preoperative planning of any shoulder arthroplasty case. A CT scan is preferred over standard plain film radiographic assessment as it provides the surgeon with a far superior appreciation of the 3D nature of the defect and allows prior identification and planning of potential challenges involved in restoring correct glenoid alignment and version and addressing glenoid bone stock.
Preoperative assessment of GBL
Preoperative assessment of GBL is critical in order to develop a plan for appropriate management. Standard plain radiographs including AP, Grashey view, as well as trans-scapular lateral and axillary views are recommended as an initial screening assessment to identify the presence of GBL, excessive glenoid medialization, and humeral head subluxation or superior migration (19). CT imaging with slice thickness <1.5 mm is recommended to accurately assess the glenoid anatomy, glenoid version, and vault size (33,34). It is important to obtain a CT scan which includes the medial border of the scapula to allow for accurate interpretation of the trans-scapular (Friedman’s) line for assessment of humeral head subluxation (35).
Generally, three lines on a CT scan are required to measure glenoid version (anteversion or retroversion): Friedman’s line, a line perpendicular to Friedman’s line, and a line between the anterior and posterior glenoid margins (the glenoid vault axis) (35). The angle of version is then measured as the angle between the line perpendicular to Friedman’s line and the glenoid vault axis (35). However, this conventional method may be impacted by the scapular shape and body, which varies from one patient to another. Matsumura et al. described another method of measuring glenoid version: the vault version method (33). In this method, the glenoid vault axis is defined as a line between the tip of the scapular vault to the center of the glenoid line (33). When utilizing this technique, Matsumura et al. reported a mean glenoid retroversion of 1.10°±3.20° in normal shoulder controls as measured by the Friedmans line, in comparison to 8.90°±2.70° using the vault method (33). Such findings suggest that the Friedman technique may actually underestimate the significance of bone loss and degree of change in version in this patient population. Despite these findings, no consensus on optimal assessment of glenoid version exists (33,34).
Computer planning software and patient-specific instrumentation (PSI)
Computer planning software and PSI may help to increase the accuracy of glenoid component position, particularly in more complex cases with significant GBL and version deformity (34). They may optimize the position of the desired glenoid implant, utilizing guides developed specifically for the patient or by providing the treating surgeon with improved spatial awareness of glenoid morphology, improving their ability to address defects with more accurate placement of standard guides (34). The aim of these tools is to prevent or mitigate malposition of the glenoid component which is known to result in decreased shoulder range of motion and increased risk of scapular notching, instability, and loosening, leading to the ultimate failure of the implant or compromise in outcome of the arthroplasty (34).
In order to utilize PSI, the surgeon must obtain a preoperative thin-cut CT scan of the entire scapula and humerus utilizing a predefined protocol. Image processing software converts obtained CT images into a precise 3D model of the patient’s scapula which is used to develop a patient specific guide which confirms to an individual patient’s anatomy. This guide then allows for precise placement of central pin for the glenoid component allowing for correction of deformity as predetermined by the surgical plan (36).
Computer-navigated instrumentation
The advancement of computer-navigated instrumentation provides a surgeon with improved ability to manage GBL by providing real-time visual feedback to guide instrument positioning. This allows for improved ability to replicate a preoperative plan, particularly in challenging cases of severe bone loss or deformity. This is achieved through using line-of-sight cameras and trackers secured to the patient, which track surgical instruments in real time providing visual feedback to the surgeon (37,38).
Compared to patient-specific guides, which require custom manufacturing and a delay in production, navigation systems are readily available immediately following preoperative planning. They offer the advantage of flexibility, allowing for intraoperative alterations to the surgical plan based on the surgeon’s discretion, if unexpected factors are encountered (38).
One specific application of computer navigation in GBL is the navigation of glenoid baseplate variable angle compression screws. This technology enables visualization of a customized trajectory into the glenoid vault, facilitating the optimization of screw length and purchase (37). By maximizing these parameters improved fixation of the implant at time zero can be achieved. While numerous studies have validated the improved accuracy and precision of glenoid baseplate implantation using navigation in RSA, the clinical benefits in terms of improved outcomes, reduced complications associated with glenoid malpositioning, and long-term implant survival remain uncertain (39,40). Therefore, further research is needed to establish the clinical advantages of navigation systems in the management of GBL.
Surgical techniques of glenoid reconstruction
The surgical technique used to address the defect will depend on the degree and location of GBL (23). As previously mentioned, bone loss is generally superior in cases of CTA, posterior in cases of primary osteoarthritis or dysplasia, and anterior in cases of chronic anterior dislocations (8). Global defects are more likely to be encountered in the revision setting. Generally, when managing any glenoid defect, the goal is to restore the joint line.
Eccentric glenoid reaming
In cases of mild GBL and version, eccentric reaming is a technique wherein the glenoid surface opposite to the site of bone loss is preferentially reamed to re-establish the natural glenoid version and correct the deformity. In patients with 5–8 mm of posterior GBL and retroversion up to 15°, this is a suitable technique to restore neutral alignment (21). However, there are limits to eccentric reaming for patients with severe GBL and irregular version. Over-aggressive reaming can reduce available subchondral support and excessively medialize the glenoid implant, which may negatively impact glenoid component stability (41). When required, bone grafting with morselized cortico-cancellous allograft or autograft can be packed into any remaining small defects after eccentric reaming has been completed to provide additional bony support and bone stock.
Glenoid bone grafting
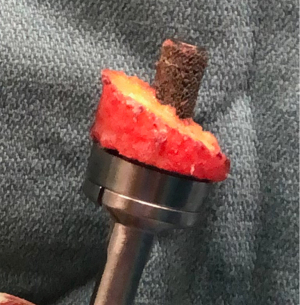
Bone grafting is often used in cases of significant bone loss where adequate version correction and secure seating of a glenoid component are difficult to attain. For larger defects, structural grafts are needed in order to stabilize the glenoid component. Both autografts and allografts are commonly used to treat GBL. The proximal humeral head provides an excellent option for local bone graft when addressing GBL (42). Once the humeral head is resected, the size of the humeral autograft can be determined based on a preoperative template. The glenoid is prepared and all remaining cartilage is removed until an appropriate bleeding bone bed is created. This can be done utilizing a combination of curet and burr. The resected humeral graft can then be secured to the glenoid with Kirschner wires for temporary fixation, while the baseplate is secured with a central post opening in the graft. Further fixation is then obtained with screws through the baseplate (refer to Figure 2) (16). In cases in which humeral head autograft is not available, such as in revision or traumatic settings, an iliac crest bone graft is another option to address GBL. Many published studies evaluating RSA with glenoid bone grafting for both primary and revision cases present satisfactory outcomes (13).
Paul et al. performed a systematic review evaluating glenoid bone grafting in primary RSA. This review comprised 11 studies involving 393 patients, and found that glenoid bone grafting in primary RSA results in excellent early-term clinical outcomes, low complication and revisions rates of 18% and 2% respectively, and an overall graft union rate of 92% (43).
Wagner et al. evaluated patients who underwent glenoid bone grafting in revision RSA (44). In this study, the implant survival rate at two and five years was 92% and 89% respectively in patients who did not experience radiographic glenoid loosening (44). Melis et al. who also evaluated glenoid bone grafting in revision RSA, reported a 76% graft incorporation rate and 8% glenoid loosening rate at mean follow up of 47 months (45).
To date, no significant postoperative differences have been found when comparing patients who receive autografts vs. allografts. Mahylis et al. and Jones et al. both compared patients who received either an autograft or an allograft in RSA, and found no significant differences in postoperative clinical and functional outcomes between the two groups (46,47). Fliegel et al. performed a systematic review on biologic graft augmentation for GBL in conversion of failed anatomic to RSA, to examine the success and failure of biologic glenoid bone grafting to address vault deficiencies (48). In this review which included 12 studies involving 200 total patients, 73 patients received an autograft, 121 patients received an allograft, and 6 patients received a hybrid autograft/allograft. The authors found no clear difference between failure rates of autograft vs. allograft to address these defects (48).
It is important to note that these studies have limitations, such as small sample sizes, and further research is necessary to draw more definitive conclusions about the effectiveness of autograft and allograft bone grafting in the setting of GBL.
Glenoid augments
Augmented glenoid components are also used to address GBL in shoulder arthroplasty. They help to secure the region of the glenoid where there is bone deficiency, which helps to avoid some of the complications seen with bone grafting such as graft nonunion, resorption, and symptomatic implant failure (49).
In the setting of RSA, Ghanta et al. performed a systematic review evaluating an augmented baseplate, which included seven studies involving 810 total patients. They reported that the use of augmented baseplates in RSA produces positive clinical and functional outcomes at early follow-up, with low revision rates. The authors concluded that augmented baseplates are a viable option to address GBL in RSA (50).
To compare glenoid bone grafts vs. glenoid augments, Lanham et al. performed a systematic review comparing outcomes of RSA using either a bone graft or augmented baseplate for the management of GBL. This review included 19 studies involving 652 total patients, and found that both options have similar overall clinical outcomes as well as complication and revision rates. Bone grafts exhibited an 11.7% complication rate and 4.5% revision rate, while augmented baseplates exhibited a 11.8% complication rate and 3.7% revision rate (51). It is worth mentioning that bone grafting and augmented baseplate have also been utilized in anatomic TSA.
A systematic review by Wilcox et al. also sought to compare the outcomes of glenoid bone grafting vs. augmented glenoid implants to address GBL in RSA. In this review, a total of 13 studies involving 919 total shoulders were included. The authors concluded that both bone grafts and augmented glenoid baseplates resulted in excellent range of motion and functional outcomes in primary RSA. They noted that the use of augmented baseplates may lead to fewer complications and revisions (52).
Custom and patient-specific technology
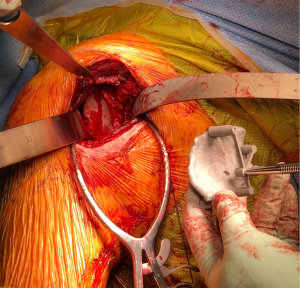
Although glenoid augments are a generic option used to treat bone loss, it is possible to create a custom glenoid baseplate specific to a particular patient’s bone loss and bony morphology (11,53). This patient-specific approach uses computer-assisted design and manufacturing technology to develop custom implants. This is particularly useful in cases of significant glenoid deformity, where the fixation of a generic glenoid baseplate may not produce optimal results. Progress in 3D printing technology gives orthopaedic surgeons the ability to manufacture implants that accurately match patient-specific glenoid morphology. As aforementioned, PSI increases the accuracy of implantation of the glenoid components (refer to Figure 3). Of note, custom-made implants are mainly recommended in cases of severe deformity, based on necessity, considering the high costs and lack of long term reported outcomes (53).
In assessing the available literature on the management of GBL, it is important to recognize the limitations of the current body of research. The strength of this review lies in providing an updated assessment of the literature and offering recommendations for GBL management. However, it is important to acknowledge that the available literature is characterized by low methodological quality and a lack of comparative studies. Consequently, direct comparisons between different treatment options are often not possible due to insufficient evidence. Long term data does not exist for many new technological advances such as navigation and patient specific instrumentation. These limitations highlight the need for further research and higher-quality studies to establish more comprehensive guidelines and comparative evaluations of various management options for GBL.
Conclusions
In summary, GBL is not uncommon in shoulder arthroplasty and can be attributed to a variety of pathologies. It is important to utilize caution when interpreting classification schemes, particularly those based on the two-dimensional appearance of the glenoid, given the complex three-dimensional nature of the glenoid. The aim of deformity correction is to optimize the implant in a near-neutral position maximizing time zero fixation of the implant. Various techniques ranging from eccentric reaming to bone and metal augmentation and custom implants are available to optimize fixation and deformity correction. Long term comparative studies are required to inform optimal management when considering bony or metal augments as well as novel biomaterials for the various deformity patters a shoulder surgeon will encounter when performing shoulder arthroplasty.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathan D. Hughes and Albert Lin) for the series “Bone Loss in Shoulder Instability and Shoulder Arthroplasty” published in Annals of Joint. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-24/rc
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-24/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-24/coif). The series “Bone Loss in Shoulder Instability and Shoulder Arthroplasty” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGuire DT, Vrettos B, Roche S, et al. Bone loss in shoulder replacement surgery: a review of current management. SA Orthopaedic Journal 2012;11:47-55.
- Seidl AJ, Williams GR, Boileau P. Challenges in Reverse Shoulder Arthroplasty: Addressing Glenoid Bone Loss. Orthopedics 2016;39:14-23. [Crossref] [PubMed]
- Gupta A, Thussbas C, Koch M, et al. Management of glenoid bone defects with reverse shoulder arthroplasty-surgical technique and clinical outcomes. J Shoulder Elbow Surg 2018;27:853-62. [Crossref] [PubMed]
- Reahl GB, Abdul-Rassoul H, Kim RL, et al. Anatomic vs. reverse shoulder arthroplasty for the treatment of Walch B2 glenoid morphology: a systematic review and meta-analysis. JSES Rev Rep Tech 2021;1:317-28. [Crossref] [PubMed]
- Menendez ME, Garrigues GE, Jawa A. Clinical Faceoff: Anatomic Versus Reverse Shoulder Arthroplasty for the Treatment of Glenohumeral Osteoarthritis. Clin Orthop Relat Res 2022;480:2095-100. [Crossref] [PubMed]
- Nerot C, Ohl X. Primary shoulder reverse arthroplasty: surgical technique. Orthop Traumatol Surg Res 2014;100:S181-90. [Crossref] [PubMed]
- Sears BW, Johnston PS, Ramsey ML, et al. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg 2012;20:604-13. [Crossref] [PubMed]
- Colasanti CA, Lin CC, Ross KA, et al. Augmented baseplates yield optimum outcomes when compared with bone graft augmentation for managing glenoid deformity during reverse total shoulder arthroplasty: a retrospective comparative study. J Shoulder Elbow Surg 2023;32:958-71. [Crossref] [PubMed]
- Singh J, Odak S, Neelakandan K, et al. Survivorship of autologous structural bone graft at a minimum of 2 years when used to address significant glenoid bone loss in primary and revision shoulder arthroplasty: a computed tomographic and clinical review. J Shoulder Elbow Surg 2021;30:668-78. [Crossref] [PubMed]
- Farron A, Terrier A, Büchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg 2006;15:521-6. [Crossref] [PubMed]
- Kyriacou S, Khan S, Falworth M. The management of glenoid bone loss in shoulder arthroplasty. J Arthrosc Jt Surg 2019;6:21-30. [Crossref]
- Friedman LGM, Garrigues GE. Management of Humeral and Glenoid Bone Defects in Reverse Shoulder Arthroplasty. J Am Acad Orthop Surg 2021;29:e846-59. [Crossref] [PubMed]
- Malahias MA, Chytas D, Kostretzis L, et al. Bone grafting in primary and revision reverse total shoulder arthroplasty for the management of glenoid bone loss: A systematic review. J Orthop 2020;20:78-86. [Crossref] [PubMed]
- Rondon AJ, Williams AA, Tzeuton S, et al. Total shoulder arthroplasty using an inlay glenoid component for glenoid deficiency: mid- to long-term follow-up of a previously published cohort. J Shoulder Elbow Surg 2022;31:2281-6. [Crossref] [PubMed]
- Song DJ, Grogan BF, Jobin CM. Anatomic total shoulder arthroplasty for severe glenoid bone loss: Still a viable option. Semin Arthroplasty 2018;29:83-6. [Crossref]
- Nicholson GP, Cvetanovich GL, Rao AJ, et al. Posterior glenoid bone grafting in total shoulder arthroplasty for osteoarthritis with severe posterior glenoid wear. J Shoulder Elbow Surg 2017;26:1844-53. [Crossref] [PubMed]
- Klein SM. RSA with Glenoid Bone Loss. In: Frankle M, Marberry S, Pupello D. editors. Reverse Shoulder Arthroplasty: Biomechanics, Clinical Techniques, and Current Technologies. Cham: Springer International Publishing; 2016:179-90.
- Wagner E, Sperling JW. Management of Glenoid Bone Loss in Reverse Shoulder Arthroplasty. Acta of Shoulder and Elbow Surgery 2017;2:28-31.
- Bercik MJ, Kruse K 2nd, Yalizis M, et al. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg 2016;25:1601-6. [Crossref] [PubMed]
- Loucas R, Kriechling P, Loucas M, et al. Reverse total shoulder arthroplasty in patients with type B2, B3, and type C glenoids: comparable clinical outcome to patients without compromised glenoid bone stock-a matched pair analysis. Arch Orthop Trauma Surg 2022;142:3687-95. [Crossref] [PubMed]
- Hendel MD, Werner BC, Camp CL, et al. Management of the Biconcave (B2) Glenoid in Shoulder Arthroplasty: Technical Considerations. Am J Orthop (Belle Mead NJ) 2016;45:220-7. [PubMed]
- Rispoli DM, Sperling JW, Athwal GS, et al. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am 2006;88:2637-44. [Crossref] [PubMed]
- Malhas A, Rashid A, Copas D, et al. Glenoid bone loss in primary and revision shoulder arthroplasty. Shoulder Elbow 2016;8:229-40. [Crossref] [PubMed]
- Sirveaux F, Favard L, Oudet D, et al. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br 2004;86:388-95. [Crossref] [PubMed]
- Habermeyer P, Magosch P, Luz V, et al. Three-dimensional glenoid deformity in patients with osteoarthritis: a radiographic analysis. J Bone Joint Surg Am 2006;88:1301-7. [PubMed]
- Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am 1983;65:1232-44. [Crossref] [PubMed]
- Hamada K, Yamanaka K, Uchiyama Y, et al. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res 2011;469:2452-60. [Crossref] [PubMed]
- Visotsky JL, Basamania C, Seebauer L, et al. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am 2004;86-A:35-40. [Crossref] [PubMed]
- Antuna SA, Sperling JW, Cofield RH, et al. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg 2001;10:217-24. [Crossref] [PubMed]
- Page RS, Haines JF, Trail I. Impaction Bone Grafting of the Glenoid in Revision Shoulder Arthroplasty: Classification, Technical Description and Early Results. Shoulder & Elbow 2009;1:81-8. [Crossref]
- Rockwood CA. The Shoulder. Saunders; 2009.
- Frankle MA, Teramoto A, Luo ZP, et al. Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg 2009;18:874-85. [Crossref] [PubMed]
- Matsumura N, Ogawa K, Ikegami H, et al. Computed tomography measurement of glenoid vault version as an alternative measuring method for glenoid version. J Orthop Surg Res 2014;9:17. [Crossref] [PubMed]
- Wylie JD, Tashjian RZ. Planning software and patient-specific instruments in shoulder arthroplasty. Curr Rev Musculoskelet Med 2016;9:1-9. [Crossref] [PubMed]
- Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am 1992;74:1032-7. [Crossref] [PubMed]
- Cabarcas BC, Cvetanovich GL, Espinoza-Orias AA, et al. Novel 3-dimensionally printed patient-specific guide improves accuracy compared with standard total shoulder arthroplasty guide: a cadaveric study. JSES Open Access 2019;3:83-92.
- Holzgrefe RE, Hao KA, Panther EJ, et al. Early clinical outcomes following navigation-assisted baseplate fixation in reverse total shoulder arthroplasty: a matched cohort study. J Shoulder Elbow Surg 2023;32:302-9. [Crossref] [PubMed]
- Schoch BS, Haupt E, Leonor T, et al. Computer navigation leads to more accurate glenoid targeting during total shoulder arthroplasty compared with 3-dimensional preoperative planning alone. J Shoulder Elbow Surg 2020;29:2257-63. [Crossref] [PubMed]
- Hao KA, Sutton CD, Wright TW, et al. Influence of glenoid wear pattern on glenoid component placement accuracy in shoulder arthroplasty. JSES Int 2022;6:200-8. [Crossref] [PubMed]
- Lohre R, Warner JJP, Athwal GS, et al. The evolution of virtual reality in shoulder and elbow surgery. JSES Int 2020;4:215-23. [Crossref] [PubMed]
- Lazarus MD, Jensen KL, Southworth C, et al. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am 2002;84:1174-82. [Crossref] [PubMed]
- Boileau P, Morin-Salvo N, Bessière C, et al. Bony increased-offset-reverse shoulder arthroplasty: 5 to 10 years' follow-up. J Shoulder Elbow Surg 2020;29:2111-22. [Crossref] [PubMed]
- Paul RA, Maldonado-Rodriguez N, Docter S, et al. Glenoid bone grafting in primary reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2019;28:2447-56. [Crossref] [PubMed]
- Wagner E, Houdek MT, Griffith T, et al. Glenoid Bone-Grafting in Revision to a Reverse Total Shoulder Arthroplasty. J Bone Joint Surg Am 2015;97:1653-60. [Crossref] [PubMed]
- Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg 2012;21:342-9. [Crossref] [PubMed]
- Mahylis JM, Puzzitiello RN, Ho JC, et al. Comparison of radiographic and clinical outcomes of revision reverse total shoulder arthroplasty with structural versus nonstructural bone graft. J Shoulder Elbow Surg 2019;28:e1-9. [Crossref] [PubMed]
- Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg 2016;25:1425-32. [Crossref] [PubMed]
- Fliegel BE, DeBernardis D, Ford E, et al. Biologic graft augmentation for glenoid bone loss in conversion of failed anatomic to reverse shoulder arthroplasty: a systematic review. JSES Rev Rep Tech 2023;3:44-8. [Crossref] [PubMed]
- Kirsch JM, Patel M, Singh A, et al. Early clinical and radiographic outcomes of an augmented baseplate in reverse shoulder arthroplasty for glenohumeral arthritis with glenoid deformity. J Shoulder Elbow Surg 2021;30:S123-30. [Crossref] [PubMed]
- Ghanta RB, Tsay EL, Feeley B. Augmented baseplates in reverse shoulder arthroplasty: a systematic review of outcomes and complications. JSES Rev Rep Tech 2023;3:37-43. [Crossref] [PubMed]
- Lanham NS, Peterson JR, Ahmed R, et al. Comparison of glenoid bone grafting vs. augmented glenoid baseplates in reverse shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2023;32:885-91. [Crossref] [PubMed]
- Wilcox B, Campbell RJ, Low AK, et al. Management of glenoid bone loss in primary reverse shoulder arthroplasty: a systematic review and meta-analysis. Bone Joint J 2022;104-B:1334-42. [Crossref] [PubMed]
- Porcellini G, Micheloni GM, Tarallo L, et al. Custom-made reverse shoulder arthroplasty for severe glenoid bone loss: review of the literature and our preliminary results. J Orthop Traumatol 2021;22:2. [Crossref] [PubMed]
Cite this article as: Al-Omairi S, Albadran A, Dagher D, Leroux T, Khan M. Glenoid bone loss in shoulder arthroplasty: a narrative review. Ann Joint 2024;9:8.