
Management of acetabular bone loss in revision total hip replacement: a narrative literature review
Introduction
Total hip replacement (THR) continues to be one of the most successful orthopedic procedures with excellent long-term outcomes, pain free motion and excellent functional improvement. With an increasing number of primary THR in younger patients, 10% to 15% are estimated to undergo revision at 20 years (1). Additionally, there is a statistically significant increase in overall number and cost of revision THR since 2002 (2). Common causes of THR failure include infection, aseptic loosening, osteolysis, and instability, all of which can occur with significant acetabular bone loss (3,4). The goal of revision THR in the setting of acetabular bone loss is establishing robust, long-term stability of a construct that recreates the native hip biomechanics. The longevity and ultimate function of the construct is affected by quantity and quality of available bone, stability of the components, restoration of the hip center, and biomechanics of the hip joint.
Proper evaluation of acetabular bone loss and meticulous preoperative planning are paramount in obtaining a good clinical result. Appropriate radiographs and use of classification system are crucial to identify bone loss patterns to guide available treatment options. Various acetabular reconstruction options have been developed and are still evolving for their respective patterns of bone loss. With each subsequent revision surgery, bone loss increases, and appropriate management of bone loss is paramount. Inaccurate identification of acetabular bony defects can result in inappropriate intraoperative fixation.
Currently, there is no consensus regarding the best option for reconstructing massive acetabular defects (5). Mid to long-term outcomes in small series for various techniques in managing acetabular bone loss show promising results. This narrative review will discuss appropriate preoperative workup and imaging evaluation for patients with acetabular bone loss who are indicated for revision surgery, summarize the most common classification systems for acetabular bone loss, and review recent literature with regard to specific surgical techniques and clinical outcomes for management of acetabular bone loss. We present this article in accordance with the Narrative Review reporting checklist (available at https://aoj.amegroups.org/article/view/10.21037/aoj-23-23/rc).
Methods
An extensive literature search was performed using PubMed and Cochrane Library for studies reporting outcomes on acetabular reconstruction for bone loss in revision THR. Articles from 2011 to 2022 were included, and citations within the selected articles were evaluated for inclusion. Complete search criteria are presented in Table 1. Studies were selected by two authors. The study data including authors, bone defect classification, number of patients, reconstruction techniques, and follow-up were extracted. Outcomes from these studies were organized according to key variables such as functional outcomes, Harris Hip Score (HHS), implant survival rate, failure rate as re-revision due to any cause and aseptic loosening and radiographic component migration (>5 mm and or >5 degree).
Table 1
| Items | Specification |
|---|---|
| Date of search | 01/30/2023 |
| Databases and other sources searched | PubMed, Cochrane Library |
| Search terms used | Acetabular reconstruction, revision total hip replacement |
| Timeframe | 2011–2022 |
| Inclusion criteria | Retrospective, prospective studies; instructional course lectures; international symposium; English article |
| Selection process | Independently selected by A.K.P., A.F.K. |
Preoperative evaluation
All patients undergoing revision THR should be thoroughly assessed for possible joint infection and associated comorbidities. Inflammatory serum markers erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin, and alpha-defensin can be ordered, and if elevated joint aspiration should be performed for bacterial culture and antibiotic sensitivities (6). Several criteria can be utilized to assess for prosthetic joint infection (PJI). Commonly used criteria are the Musculoskeletal Infection Society (MSIS) and the European Bone and Joint Infection Society (EBJIS) (6,7). Given the difficulty of diagnosing PJIs and the complexity and morbidity of treatment of PJI, diagnosis criteria are critical. Table 2 outlines the EBJIS definition of PJI (7). Prior to any revision for acetabular bone loss, the diagnosis of PJI must be ruled out.
Table 2
| Parameters | Infection unlikely | Infection likely 2 positive findings | Infection confirmed any positive finding |
|---|---|---|---|
| Clinical features | Clear reasons for prosthesis dysfunction (fracture, malposition, implant breakage, tumor) | Radiological signs of loosening within the first 5 years after implantation, previous wound healing problems, history of recent fever or bacteremia, purulence around the prosthesis | Sinus tract with evidence of communication to the joint or visualization of the prosthesis |
| CRP | <1 mg/dL | >10 mg/dL | – |
| Synovial fluid leukocyte counts | <1,500 cells/UL with PMN <65% | >1,500 cells/UL with PMN >65% | >3,000 cells/UL with PMN >80% |
| Joint aspiration fluid, intraoperative fluid, and tissue samples | All cultures negative | Positive culture | Biomarkers have positive immunoassay of alpha defensin |
| Sonification | No growth | >1 CFU/mL of any organism on sonification | Two positive samples with the same microorganism |
| >50 CFU/mL of any organism on sonification | |||
| Histology | Negative HPF | >5 neutrophils per HPF on histology | ≥5 neutrophils in ≥5 HPF |
| Nuclear imaging | Negative three-phase isotope | Positive WBC scintigraphy on nuclear imaging | Visible microorganisms |
CRP, C-reactive protein; PMN, polymorphonuclear neutrophils; CFU, colony-forming unit; HPF, high power field; WBC, white blood cells.
All previous hip surgeries in addition to implant manufacturer information are important to be identified preoperatively. This helps determine the need for explantation instruments during surgery and will identify the modular components that need to be available intra-operatively. Patients should be evaluated for chronic medical conditions especially diabetes and cardiopulmonary disorders, which may affect postoperative course in the form of infection or suboptimal rehabilitation. Massive acetabular reconstructions are often a significant physiological undertaking, so optimization preoperatively is paramount for patient safety.
Developing a plan for acetabular reconstruction and success of revision THR depends largely on understanding the quantity, quality, and location of acetabular bone loss. Radiographic assessment should include radiographs of the anteroposterior (AP) pelvis, AP and lateral views of the hip, and Judet views. The lumbosacral spine should also be evaluated for hypo or hypermobility and pelvic tilt to assist with optimal cup positioning. Internal oblique (obturator view) shows integrity of iliopectineal line (anterior column) and posterior acetabular wall. External oblique (iliac view) shows integrity of ilioischial line or Kohler’s line (posterior column) and anterior acetabular wall. Radiographs are useful to assess the teardrop and ischial tuberosity lysis, which is utilized for Paprosky classification (6). Templating should be performed, and preoperative leg length discrepancy (LLD) and location of hip center of rotation (COR) should be recorded. Radiographs will also allow for assessment of bone quality, which will help determine the mode of fixation.
Appropriate radiographs should provide sufficient information about bone loss; however, there should be a low threshold to obtain a computed tomography (CT) scan to further characterize severe acetabular bone loss and optimizing reconstruction plans. 3D CT is an excellent supplement if radiographs fail to provide proper assessment of degree and location of bone loss. Magnetic resonance imaging (MRI) is infrequently needed but may be useful to evaluate soft tissue abnormalities such as metallosis or pseudotumor formation or the quality of the abductor complex. CT angiography should be ordered in cases of medial wall defects, protrusion, and pelvic discontinuity.
Classification system
Classification of acetabular bone defects is useful to determine the appropriate technique for stable fixation. The surgeon should be comfortable with a system to consistently classify bone loss and successful planning. Four classification systems have been used for acetabular bone loss: the American Academy of Orthopaedic Surgeons (AAOS) classification (8), Ghanem & Andreas classification (9), the Gross classification (10), and the Paprosky classification (11). The AAOS classification describes bone loss by pattern and location, but it does not specify the size of the defect. The pattern is described as segmental (Type I), cavitary (Type II), or combined (Type III) defects. Type IV defects involve pelvic discontinuity, and Type V defects indicate hip arthrodesis (8). Segmental and cavitary defects can be further classified by location as a peripheral (superior, posterior, or anterior) defect or central (medial) defect. Given that this classification does not quantify bone loss, its application is limited for treatment planning. Ghanem et al. described a classification that is mainly based on intraoperative assessment of the bone loss to determine whether 3-point fixation of an acetabular cup is possible and suggests constructs to address the defect based on this assessment (Table 3) (9). The Gross classification describes acetabular bone loss as having contained defects or uncontained defects in addition to pelvic discontinuity as a separate type in the classification (Table 4) (10). The authors of this review prefer to use the Paprosky classification. This system is widely accepted and provides a basis for implant selection. Using this classification, the location and extent of bone loss are predicted based on preoperative radiographs, and reconstruction options can be determined according to the classification (Table 5) (11). For example, Paprosky 3B defects are the most severe, where supporting bone loss is greater than 60% with significant superior-medial migration of the hip COR. Antiprotrusio ilioischial cage constructs have been used to reconstruct these defects. Sporer outlined a treatment algorithm for acetabular revision surgery based on the Paprosky classification (Table 6) (12).
Table 3
| Classification | Acetabular bony configuration |
|---|---|
| Type I | Possible 3‑point fixation within the boundaries of the acetabular wall, hemispherical configuration of the acetabulum |
| Hemispherical (preferably cementless; cemented only in case of adequate cancellous bone structure and absence of bone defects) ± allogenic cancellous bone | |
| Type II | Possible 3‑point fixation within the boundaries of the acetabular wall, cavitary/oval configuration of the acetabulum |
| Cementless oval cups or spherical cups with augmentation parts ± allogenic cancellous bone | |
| Type III | Impossible 3‑point fixation within the boundaries of the acetabular wall, cavitary configuration of the acetabulum with severe bone loss or pelvic discontinuity |
| Cementless acetabular cup with cranial strap ± iliac stem + allogenic cancellous bone or cup-cage system + allogenic cancellous bone | |
| Type IV | Impossible 3‑point fixation within the boundaries of the acetabular wall, pelvic discontinuity with major bone loss and destruction of iliac bone |
| Custom triflange acetabular components |
Table 4
| Type | Description of acetabular bone loss |
|---|---|
| I | Mild loss of acetabular bone stock |
| II | Contained loss of acetabular bone stock, intact rim and column |
| III | Uncontained loss of acetabular bone stock (≤50% host bone) |
| IV | Uncontained loss of acetabular bone stock (>50% host bone) |
| V | Contained loss of acetabular bone stock with pelvic discontinuity |
Table 5
| Types | Femoral head center of rotation | Tear drop lysis | Ilioischial (Kohler’s line) integrity | Ischial tuberosity lysis | Anterior & posterior columns | Prosthesis coverage by host bone | Implant types |
|---|---|---|---|---|---|---|---|
| I | None | Intact | Intact | None | Intact | >70% | Uncemented porous coated hemispherical cup with multiple screws |
| IIA | Mild <3 cm | Intact | Intact | None | Intact | 70% | Uncemented porous coated hemispherical cup with multiple screws |
| IIB | Moderate <3 cm |
Intact | Intact | Mild | Intact | 50–70% | Uncemented porous coated hemispherical cup with multiple screws |
| IIC | Mild <3 cm | Moderate | Disrupted | Mild | Intact | <50% | Uncemented porous coated hemispherical cup with multiple screws and bone grafting |
| IIIA, up & out | Severe >3 cm | Moderate | Intact | Moderate | Superolateral defect | 30–60% rim defect | Uncemented, porous hemispherical shell, porous metal augments or allograft, screws, and cement |
| IIIB, up & in | Severe >3 cm | Severe | Disrupted | Severe | Superomedial defect | >60% host bone loss | Uncemented, porous shell, modular porous metal augments/allograft, screws, reconstruction cage or CTAC |
CTAC, custom triflange acetabular component.
Table 6
| Classification types | Affected hip COR superior migration | Press fit stable trial cup possible | Biologic fixation with hemispherical shell possible | PD | Fracture healing potential | Intact illioischial line | Implant types |
|---|---|---|---|---|---|---|---|
| Type I & IIA mild cavitary contained bony defect | <2 cm | Yes | – | – | – | Yes | Hemispherical shell |
| Type IIB moderate bony defect with segmental rim loss <30% | |||||||
| Type IIC protrusio | <2 cm | Yes | – | – | – | No | Medial bone impaction grafting morselized allografts, hemispherical shell |
| Type IIIA | >2 cm | No | Yes | – | – | – | 1. Jumbo cup |
| 1. Spherical acetabular defect | 2A High hip COR hemispherical shell | ||||||
| 2. Oblong acetabular defect | 2B Structural allograft with hemispherical shell | ||||||
| 2C TM shell with modular metal superior augment | |||||||
| Type IIIB without PD | >2 cm | No | No | No | – | – | 1. Biologic fixation: |
| A TM shell & modular metal augments | |||||||
| B Patient specific CTACs | |||||||
| 2. Non-biologic fixation-structural allograft with cage | |||||||
| Type IIIB Acute PD | >2 cm | No | No | Yes | Yes | – | 1. Acetabular compression plate, cage & allografts |
| 2. Trabecular metal shell | |||||||
| Type IIIB Chronic PD | >2 cm | No | No | Yes | No | – | 1. Acetabular distraction |
| 2. 3D printed TM shell & modular metal augments | |||||||
| 3. Patient specific CTAC |
COR, center of rotation; PD, pelvic discontinuity; TM, trabecular metal; CTAC, custom triflange acetabular component.
Recently, two additional classification systems have been proposed. Wirtz et al. proposed a system based on the integrity of the rim and supporting structures, and the authors reported the inter- and intra-rater reliability to conclude that their classification system was intuitive, reliable, and reproducible. This classification is summarized in Table 7 (13). Walter et al. developed an intraoperative classification method based on intraoperative stability—the Stability Classification for Acetabular Replacement (SCAR) (14). This classification consists of five categories of percentage of containment, and a summary of the classification system is outlined in Table 8.
Table 7
| Type | Defects | Implant choice |
|---|---|---|
| I | Contained cancellous bone defect intact rim | |
| A | Random cancellous bone defect | Pressfit cup/screw-in cup. Impaction bone grafting medial and superomedial acetabulum |
| B | Superomedial wall defect | Pressfit cup/screw-in cup. Impaction bone grafting medial and superomedial acetabulum |
| C | Medial wall defect with intact anterior & posterior wall integrity | Pressfit cup/cup and cage/modular cage/screw-in-cup. Impaction bone grafting medial and superomedial acetabulum |
| II | Noncontained nonstructural acetabular rim defect <10 mm vertically with cancellous bone defects | Metal-augmentation of defect through |
| A | Superolateral defect | A: Augment-and-cup/augment-and-(modular)-cage/oblong cup/cranial socket system |
| B | Posterior column deficiency | B/C: Additional flanges and/or iliac peg Impaction bone grafting medial and superomedial acetabulum |
| C | Combination of A & B | |
| III | Noncontained, structural acetabular rim defect >10 mm | Metal-augmentation of defect with additional flanges through: augment-and-(modular)-cage impaction bone grafting medial and superomedial acetabulum |
| A | Superolateral defect | |
| B | Posterior column deficiency | |
| C | Combination of A & B | |
| IV | Ilio-ischial bone stock disruption with nonsupportive anterior & posterior columns | |
| A | Supportive superior bone stock | Iliac-ischial plating with: augment-and-(modular)-cage, oblong cup/cranial socket system with iliac peg and flange, impaction bone grafting medial and superomedial acetabulum |
| B | Nonstructural superior rim defect <10 mm vertical | Augment-and-(modular)-cage, oblong cup with iliac peg and flanges, Custom individualized Monoblock pelvic replacement with tripolar cup system (dual mobility), Impaction Bone Grafting Medial and Superomedial Acetabulum |
| C | Structural superior rim defect >10 mm & pelvis discontinuity | Custom individualized monoblock pelvic replacement with tripolar cup system (dual mobility) impaction bone grafting medial and superomedial acetabulum |
Table 8
| Type | Acetabular defect | Implant options |
|---|---|---|
| I—Stable, 100% Containment | Stable situation. Complete acetabular containment. Medial wall and rim intact. Minimal osseous defects | Standard press fit cups. No grafts required |
| II—Stable >66% Containment | Overall stable situation. >2⁄3 rim intact, containment >2⁄3. Posterior, superior, or anterior defect | |
| A | Biological solution bone graft supplementation (filler graft) or impaction bone grafting and standard cup (biological solution) | |
| B | Special devise oblong screws, jumbo cup without bone graft augmentation (technological solution) | |
| III—unstable <66% containment | Unstable situation. Cup migration rim not intact. Superior anterior superior posterior defects. <2⁄3 acetabular containment | |
| A | Standard cups + augments (bone/metal) combined solution | |
| B | Special devise with plate and screws, jumbo cups (monoblock solution) | |
| IV—unstable < 66% containment and PD | Unstable situation with trans-acetabular fracture, both pillars affected | Special devise with plate/screws, iliac peg required (cages, burch schneider) (monobloc solution) |
| V—total acetabular osteolysis | Unstable situations, massive bone deformities, pelvic discontinuity, massive osteolysis | Custom-made devices monobloc solution |
PD, pelvic discontinuity.
Literature review
Twenty-one studies were selected by two authors based on the search criteria for this narrative review. Of all studies, follow-up ranged from 2 to 17 years. The majority of the studies used the Paprosky classification to describe acetabular bone loss. These studies demonstrated improved postoperative patient reported outcomes based on various reconstruction techniques. Table 9 reports the implant survival, revision rate, and radiographic migration for the respective studies.
Table 9
| Type of acetabular reconstruction techniques | Author | No. of hips | Bone defects | Mean F/U duration, years | HHS pre-op | HHS final, F/U | Implant survival rate (%) | Complications (%) | Revision for any cause (%) | Revision for aseptic loosening (%) | Radiographic migration >5 mm or >5 degree (%) | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cementless hemispherical cup | Paxton et al. (15) | 32 | AAOS III/IV | 4.5 | 52.6 | 87.3 | 91 | 14 | 9 | 9 | 11 | 3/37 |
| Jumbo cup | Zhang et al. (16) | 63 | Paprosky type 2/3 | 5.7 | 46 | 83 | 93 at 15 years | 6.3 | 6.3 | |||
| Bone impaction grafting mesh cemented cup | van Egmond et al. (17) | 27 | AAOS III/IV, Paprosky 2B/3B | 8.8 (range, 3–14) |
55 | 72 | 88 at 10 years | 48 | 11 | 18.5 | ||
| Schreurs et al. (18) | 33 | Paprosky 1/3B | 17 | 55 | 74 | 70 | 30 at 15 years | 10 at 15 years | 23 | |||
| Extended ischiopubic fixation porous metal augments and cementless cup | Huang et al. (19) | 26 | Paprosky type 2/3B | 4 | 36 | 81 | 100 at 4 years | 0 | 0 | 0 | 0 | |
| TM cup-cage | Kosashvili et al. (20) | 26 | Paprosky type IIIB | 3 | 75 | PD healing, 89 | ||||||
| Amenabar et al. (21) | 67 | Paprosky 3A/3B | 6 | 93 at 5 years, 85 at 10 years | 12 | 8.5 | 5.6 | 0 | ||||
| Custom 3D printed titanium cup & augments | Durand-Hill et al. (22) | 20 | Paprosky type 2/3 | 2 | 95 at 2 years | 1 | 5 | |||||
| Zhang et al. (23) | 31 | Paprosky type 2/3 | 2 | 40 | 69 | 100 at 2 years | 0 | 0 | 0 | 0 | ||
| TM cup augments, pelvic distraction | Sporer et al. (24) | 20 | Pelvic Discontinuity | 4.5 | 95 | 20 | 5 | |||||
| TM cup & TM augments | Sporer (12) | 12 | Paprosky type 3A/3B | 2.5 | Average Postel-Merle d’Aubigné 10.3 | 6 | ||||||
| Custom triflange acetabular component, CTAC | DeBoer et al. (25) | 20 | Paprosky type 3A/3B | 10 | HHS 80 | PD healing, 90 | 0 | 0 | ||||
| Scharff-Baauw et al. (26) | 50 | Paprosky type 3B | 2 | OHS 52.5 | OHS 30 | 100 at 2 years | 16 | 0 | 0 | 0 | ||
| Taunton et al. (27) | 57 | Paprosky type 3A/3B | 5.4 | 75 | PD healing, 81 | 30 | 1.8 | |||||
| Berasi et al. (28) | 24 | Paprosky 3B | 5 | 42 | 65 | 83 | 16 | 17 | ||||
| Winther et al. (29) | 39 | Pap 3B (PD) | 5.1 | 81 | 94 at 10 years | 5 | 21 | |||||
| Monoflanged custom made acetabular component, CMAC | Walter et al. (30) | 58 | Paprosky type 3A/3B | 5 | HHS 19.5 | HHS 59.8 | 72.4 | 24 | 36.2 | 2.7 | ||
| von Hertzberg-Boelch et al. (31) | 14 | Paprosky type 3A/3B PD | 3 | WOMAC 63.89 | WOMAC at 1 year 29.45 | 70 at 3 years, 60 at 5 years | 14.3 | 28.6 | 14.3 | 21 | ||
| Fröschen et al. (32) | 68 | Paprosky type 3A/3B | 3.5 | HHS 21 | HHS 61 | 82.7 at 3 years, 77 at 5 years | 34.4 | 36.7 | 2.9 |
F/U, follow-up; HHS, Harris Hip Score; pre-op, preoperative; AAOS, American Academy of Orthopaedic Surgeons; PD, pelvic discontinuity; TM, trabecular metal; CTAC, custom triflange acetabular component; OHS, Oxford Hip Score; CMAC, custom monoflange acetabular component; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Surgical management and outcomes
Restoration of the COR of the hip joint by managing bone defects with the planned acetabular reconstruction to achieve a stable and durable construct is the mainstay of revision THR. Various surgical techniques are based on acetabular bone loss, whether defects are small/contained or larger/non-contained (33). Depending on the surgeon’s own comfort and experience, a particular surgical approach for adequate exposure, explantation, and subsequent reconstruction of acetabulum is determined. However, due to the circumferential acetabular access required for safe revision surgery, the posterolateral approach is most often utilized for revision THR due to the exposure and extensile nature. Different types of trochanteric osteotomies can be performed to facilitate femoral head dislocation, acetabular exposure, and if needed, femoral component removal during hip revision surgeries. Femoral osteotomy is divided into 3 categories based on the site and length of trochanteric osteotomy, and integrity of the gluteal vastus soft tissue sleeve: standard single plane, trochanteric slide, and extended trochanteric osteotomy. Regardless of the planned approach or osteotomy, complete acetabular rim exposure is absolutely necessary for efficient extraction of components without causing harm or further bone loss. Osteotomes, saws, and revolving blade cup extracting devices can aid the surgeon in safe removal of implants. Ideally, the implants can be removed without causing further bone loss. Once exposure is obtained and acetabular components are removed, any fibrous tissue should be removed with a Cobb and rongeurs to expose the bone to facilitate biologic fixation. At this point, the bony bed should be carefully scrutinized to determine whether the planned reconstruction technique will attain stable fixation with the available bone. The following sections will describe common reconstruction techniques for managing acetabular bone loss and review their respective outcomes in recent literature.
Uncemented hemispherical porous coated acetabular shells
Uncemented porous coated acetabular cups are commonly utilized prostheses in revision settings, especially in Paprosky type I and II bony defects with segmental, partial rim loss and intact columns. In conjunction with modular porous metal augments and supplemental screws, these acetabular shells provide immediate component stability and long-term biological fixation through osseointegration. Occasionally the hip center can be raised in order to obtain adequate initial fixation. If partial rim loss is encountered, a hemispherical cup can still provide stable, initial fixation if a “pinch-fit” can be obtained between the anterior and posterior columns. Morselized bone graft can help restore bone loss in combination with uncemented hemispherical porous coated shells. Gaffey et al. observed improved implant survival in cementless acetabular components achieving osseointegration at 15 years (34). Some studies have reported 90% midterm survivorship at 5 to 10 years with aseptic loosening as the end point. Della Valle et al. and Park et al. reported 97% and 95% cup survivorship at 15 and 20 years of follow-up respectively in their cohort of revision THR patients with uncemented hemispherical cups (35,36). Paxton et al. showed 91% implant survival rate and 87 postoperative HHS with cementless hemispherical acetabular component at mean follow-up duration of 4.5 years in cases of massive osteolysis—at least 4 cm2 defect visualized on any single radiographic view (15). These cases were primarily AAOS III defects (91%). Current literature supports excellent longevity and improvement in outcome scores when uncemented porous coated acetabular cups are used to achieve stable initial fixation.
Jumbo cups
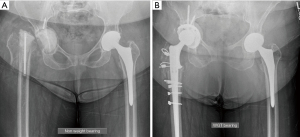
Using a cementless jumbo cup defined as ≥66 mm for men and ≥62 mm for women (10 mm larger than the mean cup diameter usually used for primary THR) is a straightforward, effective technique for treating extensive bone defects during acetabular revision (37). The jumbo cup insertion technique is similar to that of a standard hemispherical cup and thus relatively simple in comparison to other acetabular revision constructs. It also provides maximum surface contact between the component and the host bone given the large surface area and thus reduces the need for bone grafting. The jumbo cup may be associated with elevated hip COR (38). Much like the standard hemispheric cups above, jumbo cups can be bolstered with a variety of porous metal augments. Wedge shaped augments can supplement an area of fixation in an elliptical void, buttress style augments can be used to provide a strut against the metal socket, and even medial augmentation can be considered in specific cases. von Roth et al. found Harris-Galante style jumbo cups had 83% survival from any revision at 20 years (37). In another series using mainly trabecular metal cups (Zimmer, Warsaw, IN, USA), at 15-year long-term follow-up, Zhang et al. demonstrated 93% implant survival for 63 Jumbo cups implanted in patients with Paprosky type II/III acetabular bone loss (16). These patients also had an increase in mean postoperative HHS of 83. Figure 1 shows preoperative (Figure 1A) and postoperative (Figure 1B) radiographs of a second stage revision for PJI. The massive bone loss of the right acetabulum was managed with robotically-assisted jumbo cup only and retroacetabular bone grafting with morselized bulk femoral head allograft. Follow-up radiographs at 6 months show a stable construct (Figure 1B).
Bone impaction grafting with cemented cup
Impaction grafting with cemented polyethylene liner has demonstrated good results in contained, cavitary and some segmental acetabular bony defects. Bone cavities are filled and tightly packed with cancellous bone. Allogenic bone graft is often utilized to reconstitute acetabular bone loss. More studies with long-term follow-up are needed to evaluate bone graft incorporation with various adjuncts. In the setting of combined cavitary and segmental structural bony defects, impaction grafting can be protected with metal mesh before polyethylene liner is cemented. In pelvic discontinuity cases, internal fixation with reconstruction plating should be done prior to bone grafting. Busch et al. has reported 85% and 77% survival rate at 20 and 25 years respectively, after impaction grafting with cemented cup with aseptic loosening as the end point (39). Long-term results with impaction bone grafting have shown good healing of bony defects. However, availability of adequate amount of bone graft and graft resorption are some of the major challenges associated with impaction grafting. van Egmond et al. followed 27 patients for 9 years who had undergone bone impaction grafting, mesh and cemented cup for Paprosky type IIB/IIIA defects (17). They demonstrated 88% implant survival at 10 years with 12% (3/25) requiring revision of the cup for cup failure—one unstable cup, one septic loosening, one aseptic loosening. HHS improved from 55 preoperatively to 72 postoperatively. Radiographic component migration was 18%. Although this is a small sample size, this study shows promising mid-term results at 10 years with bone impaction grafting of large acetabular defects—Paprosky 2B, 3A and 3B (17). In another study on bone impaction grafting with a cemented cup, Verspeek et al. reported a 15-year risk of failure of 27% for re-revision of any component and a failure rate of 10% at 15 years for re-revision for aseptic loosening (40).
Structural bulk allograft
Structural bulk allograft can be used to restore host acetabular bone. It provides immediate structural support and mechanical stability for uncemented acetabular fixation before host bone ingrowth to the implant surface. Femoral head allograft is most frequently utilized for this purpose. However, bone resorption, infection and component loosening are major drawbacks with the use of structural allograft in revision THR. With the advent of newer generation modular highly porous coated metal augments, there is significant reduction in the use of structural allograft. This technique has thus become more historic, as results of the hemispheric and jumbo cups with metal augmentation has fewer drawbacks and superior long-term outcomes. Lee et al. retrospectively reviewed 74 patients treated with minor column shelf structural allograft for uncontained host acetabular bone deficits measuring 30% to 50%. Minimum clinical follow-up was 5 years. With re-revision for aseptic loosening as an end point, cup survivorship at 15 and 20 years was 67% and 61%, respectively, and graft survivorship was 81% (41).
Ring and cage reconstruction
Acetabular dome reinforcement ring in combination with antiprotrusio cages, mostly Bursch Schneider cages, spanning from ilium to ischium have been widely used in the past to reconstruct severe acetabular bone deficits. Screws are used to secure the cage to the ilium and the ischium. This construct protects morselized or structural bone grafts during incorporation and healing stages. The polyethylene liner can be cemented in the cage for proper inclination and retroversion while the cage is placed to obtain the most stable fixation to the pelvis. Major setbacks of the cages are premature breakage, fatigue fracture, flange breakage, loosening and eventually failure of revision THR with complications around 50–60% (29). Additionally, ring and cage constructs rely on screw and cement fixation rather than biological fixation, which may influence long-term survivorship. Goodman et al. reported 76% success for revision free, stable acetabular reconstruction rings with average follow-up of 4.6 years and the longest follow-up of 17.8 years (42). Ring and cage constructs are not a suitable option for younger age group given complications, demanding technique, and lack of biologic fixation compared to other techniques. However, it can still be considered as an option for low-demand elderly population with massive acetabular bony deficiency and lower life expectancy.
Cup and cage reconstruction
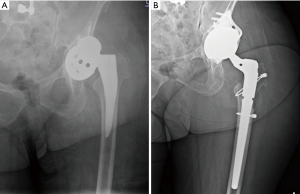
The cup-cage construct has emerged as a treatment option for severe acetabular bone defects, Paprosky Type IIIA and IIIB, and chronic pelvic discontinuity. This construct consists of a highly porous metal cup secured with screws, an ilioischial cage, and a cemented liner. The cage provides initial stability to the cup by protecting it from mechanical forces, which protects the cup to allow bone ingrowth and biologic fixation contributing to long-term stability of the entire construct (21). The uncemented porous metal acetabular shell with or without modular metal augments can be fixed with host bones with multiple supplemental screws. An antiprotrusio cage is then placed inside the uncemented shell spanning pelvic discontinuity from ilium to ischium. The ilioischial cage provides immediate mechanical strength and stability across severely compromised acetabulum with pelvic discontinuity while borrowing time for biologic fixation of uncemented shell and augments with the host bone. This technique improves on the limitations of non-biologic with ring and cage constructs by incorporating uncemented porous coated hemispherical shell into the construct. Full ilioischial cages as well as “half-cages” where the ischial flange is removed have both shown promising results. Figure 2 shows a chronic discontinuity with a failed jumbo cup (Figure 2A) that was treated with a cup-cage, half-cage construct (Figure 2B) with the ischial flange removed.
Although some studies have reported encouraging results with cup-cage constructs, but they had small number of study patients with short-term follow-up duration. Kosashvili et al. reviewed a consecutive series of 26 acetabular reconstructions with a cup-cage construct in 24 patients with pelvic discontinuity (20). The mean follow-up was 44.6 months (range, 24 to 68 months). Failure was defined as component migration of >5 mm. Twenty-three hips demonstrated no clinical or radiographic evidence of loosening at final follow-up (88.5%). The mean HHS improved from 46.6 points (range, 29.5 to 68.5 points) to 76.6 points (range, 55.5 to 92.0 points) at 2 years (P<0.001). They also noted a 89% healing rate of pelvic discontinuity at 3 years. Amenabar et al. reported 93% implant survival at 5 years and 85% at 10 years in their series of trabecular metal cup-cage constructs (21).
Oblong cup reconstruction
Oblong cup reconstruction is another customized uncemented shell for matching acetabular bone loss in Paprosky type IIB and IIIA. It’s mainly indicated for superior rim defects reconstruction, which now has largely been overtaken by uncemented Shell with Modular metal augments due to better flexibility of intraoperative acetabular reconstruction in revision THR.
Pelvic distraction technique
Pelvic discontinuity may occur in cases of severe osteolysis. Stable fixation of the acetabular prosthesis becomes difficult due to separation and motion between the superior and inferior hemipelvis. Acetabular distraction has shown reliable and durable fixation in pelvic discontinuity. Pelvic distraction technique is utilized to bridge the discontinuity and primary bone healing of the gap. Sporer et al. in their series of chronic pelvic discontinuity treated by acetabular distraction with porous tantalum components, demonstrated a predictable pain relief, durability, survivorship, improved function, and decreased mechanical failure (24). Extra acetabular distraction is used for peripheral or lateral distraction and central or medial compression at the discontinuity. Intra- and extra-cavitary defects are reconstructed by augments. After removal of implants and fibrous tissue debridement, acetabulum is first distracted from within to assess mobility. Independent motion of the superior and inferior segments is diagnosed as pelvis discontinuity. Acetabular bone loss defects are evaluated for proper metal augments selection and placement. The basis of acetabular distraction technique is to offer stable anterosuperior and posteroinferior column fit for a porous metal shell. Any intracavitary defects in the form of anterosuperior and posteroinferior column loss are first reconstructed by metal augments to provide primary construct stability prior to implantation of the shell. An augment is also used as supplemental fixation to fill extra cavitary defects due to posterosuperior bone loss if needed. Augments are first secured to the host bone with multiple screws. The acetabulum is maximally distracted via an extra-acetabular distractor and reverse reaming is performed until proper press fit between the anterosuperior and posteroinferior columns is obtained. Defects should be filled with cancellous autograft and porous tantalum acetabular shell is inserted, which is then united to the augments with cement at the augment-cup interface. The cup is fixed to the host bone by screws into the ilium, ischium and pubis. The liner is then cemented in the proper inclination and anteversion (43). Attempts are made to maximize the amount of host bone contact with the augments and acetabular component to encourage biologic fixation. Particulate graft is placed into any remaining cavities before the revision shell is impacted into place. The hemipelvis can also be distracted by placing a porous tantalum acetabular component 6 to 8 mm larger than the last reamer and continuously applying an inferiorly directed stress towards the ischium. Distraction provides press fit and initial mechanical stability of the cup until multiple screws are placed. Eleven of the 20 patients received augments in the series by Sporer et al. (24). One augment was used in eight patients, either in the anterior-superior region (six) or in the posterior-superior region. In three of these 11 patients, two augments were used to provide secure points of fixation for the socket in anterior-superior and posterior-inferior areas. Two patients with deficient abductors had a constrained liner, nine patients had a tripolar (unconstrained) articulation due to a retained femoral component, six had a 40-mm head size, one had a 36-mm head size, and two had a 32-mm head size. At 4.5 years follow-up, 95% implant survival with 20% complication rate was observed (24).
Uncemented acetabular component with modular porous metal augments
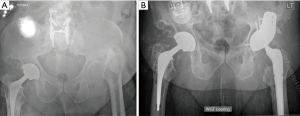
Uncemented porous acetabular shells with modular porous coated metal augments are utilized in severe structural bony loss. The porous metal augments are fixed to remaining acetabular bone with screws to supplement deficient walls and rim. The metal augments act as the new acetabular wall to support and provide mechanical stability to allow for bone ingrowth of the cup. Once implanted, metal augments are cemented to the acetabular shell to unitize the construct. Acetabular trial should be done with loosely placed metal augments before screw fixation. It’s hard to trial and estimate actual shell size after metal augments are fixed. Sporer et al. reported satisfactory outcomes in terms of biological ingrowth with uncemented acetabular shell reinforced with modular porous metal augments (24). Modular porous coated metal augments have been proven as more reliable long-term options than bulk allograft in acetabular bone loss management in revision THR. Although augments come with a higher cost than allograft, they can provide excellent anterosuperior to posteroinferior fixation that is crucial for stable socket fixation. They are also useful in posterior wall defects and other defects of the acetabular rim. They help optimize host bone-implant contact and can downgrade defects from more severe to less severe while maintaining the optimal hip center. They can convert an uncontained defect to a contained defect and decrease the overall volume of acetabular bone loss. A disadvantage of augments is that they do not restore bone in cases in which re-revision may be necessary (5). Figure 3 shows preoperative radiograph of a previous PJI status post static spacer with substantial posterior superior bone loss. This bone defect was managed with jumbo cup and buttress porous metal augmentation. Huang et al. studied cohort of 26 patients with Paprosky type II/III bone defects who were reconstructed with extended ischiopubic fixation, porous metal augments and cementless cup. They reported 100% survival without re-revision or complications at 4 years of follow-up. HHS improved from 36 preoperatively to 81 postoperatively (19).
Custom-made titanium augments have been developed by 3D printing technology. They have advantages of the porous metal augment with added accuracy. Benefits of patient specific custom-made augments are precise reaming of the acetabulum, preserving bone stock, matching the bone defects, granting adequate initial component stability. They can reconstruct the hip joint COR and restore the hip biomechanics. It offers personalized screw trajectories and precise length, reducing risk to neurovascular bundle. Acetabular components supported with custom-made 3D-printed augments is a useful method to bridge severe bone deficiencies. Zhang et al. reported favorable radiologic results and clinical outcomes with custom 3D-printed augments (23). Durand-Hill et al. reported on the 3D-CT-measured accuracy of placement of custom 3D printed titanium components in 20 patients with large acetabular defects (22). They found all components (100%) were positioned within 10 mm of planned COR. Ninety-five percent components were rotated by less than 10°, and 58% components were positioned within 5° of planned cup inclination and anteversion angle. One periprosthetic fracture was reported. Their study was largest study in which postoperative 3D-CT measurements and clinical outcomes of custom-made acetabular components have been assessed. They concluded that accurate pre-op planning and the adoption of custom 3D printed implants show promising results and may be valuable option in complex hip revision surgery (22).
Custom monoflange acetabular component (CMAC)
As proposed by Walter et al., CMAC demonstrates similar clinical outcome parameters and survival rates as Tri-flanged CMAC but with superior biomechanical features, bone ingrowth, and restoration of Anatomical COR, and therefore a solid alternative treatment option and implant design. They found no statistically significant difference in HHS, visual analog scale (VAS), survival, and revision rates between Triflanged and Monoflanged CMAC. They reported laterization and cranialization of anatomical COR in Triflanged CMAC patients due to the effects of triangulation and shifting of implants during screws tightening (14).
Fröschen et al. concluded that custom-made acetabular components are encouraging choice to restore COR and to achieve a mechanically stable reconstruction adapted to the individual acetabular bone loss in revision total hip arthroplasty (32). An acceptable survival rate and significantly improved clinical function can be achieved with CMACs. However, complication rates are high for all reconstructive options. The patient and the surgeon must be aware of the possible complications of the described method and must know how to handle them (32).
von Hertzberg-Boelch et al. described templating steps for a CMAC with optional stem for intra- and extramedullary iliac fixation for a Paprosky IIIA defect (31). First, assessment and 3D visualization of the defect situation with and without subtraction of the implant is performed (14). CT-based estimation of LLD respecting pelvic tilt and joint contractures is done. In the final steps, virtual reconstruction of the hip COR by positioning a standard acetabular component of a specific size at the anatomical COR is done. In Monoflanged CMAC, large segmental iliac defect is filled by the implant’s metallic Monoblock assembled socket. Screws are positioned in areas of the pelvis with intact host bone with a recommendation for their length in millimeters. An additional intramedullary press-fit stem can be considered to enhance the primary stability. They advocated that CMACs iliac fixation is less invasive compared to three-point fixation for triflanged custom acetabular components because it requires less preparation at the ischium. It can also routinely be implanted in supine position, which optimizes LLD. Preoperative CT-based 3D planning in CMAC yields reproducible results for leg length and hip COR. Iliac intra- and extramedullary fixation allows soft tissue-adjusted hip joint reconstruction and improves hip function. However, failure rates are high with periprosthetic infection being the main threat to successful outcome (31).
Fröschen et al. utilized CMACs as part of a two-stage procedure in patients with severe periacetabular bone loss. Preparation of the implant site is done based on preoperative planning with augmentation of bone defects as far as possible. Primarily stable anchoring with 2 angle-stable pole screws in the ilium, an optional pole screw in the pubic bone for determination of COR, and stabilization screws in the iliac wing are used. Dual mobility cup are preferred for the soft tissue tension and intraoperative stability. In their series of 47 monoflanged CMACs for Paprosky type III defect, PJI was the main complication with subsequent need for implant removal in 9 of 10 cases. HHS improved from 21.1 to 61.5 points. X-ray imaging displayed an angle of inclination of 42.3°±5.3°, and an anteversion of 16.8°±6.2° (32,44).
Custom triflange acetabular component (CTAC)
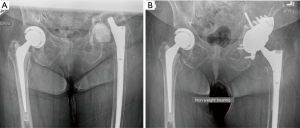
CTAC is a novel acetabular bone loss management option. CTAC is a patient specific custom-made acetabular implant that typically has plans for rigid fixation to the ilium, ischium and superior pubic ramus with screws in order to bridge across the bony defect or pelvic discontinuity. A 3D CT scan of the pelvis is obtained. The defect and structural host bone is identified on CT scan and a custom model is created to fill the defect. Screw trajectories can be planned as well. Custom drill guides can be provided to execute the plan and ensure accurate screw placement. The total process takes 4–6 weeks of planning and manufacturing. Figure 4 shows preoperative radiographs of a bailed revision arthroplasty for discontinuity with resultant massive acetabular bone loss one year later. CTAC was indicated in this case (Figure 4A). The proposed advantages of CTACs are the ability to customize and individualize the implant to the defect restoring the acetabular anatomy, choosing the optimal COR, and optimizing host bone contact area and osseointegration (Figure 4B). CTACs can provide initial implant stability due to optimized CT-guided dome and flange screw insertion (29). Drawbacks of CTAC are extensive, lengthy procurement time and high costs. However, given the implants required to achieve construct long-term stability in the setting of massive bone loss without a custom implant, the costs may be similar. Taunton et al. reported equivalent costs of the custom triflange implants and a Trabecular metal cup-cage construct: $12,500 and $11,250, respectively (27).
Walter et al. in their series of 58 patients (37 Monoflanged CMAC and 21 Triflnaged CMAC) for 5 years follow-up reported overall implant survival of 72.4%, all cause revision 36.2%, complication 24% mostly PJI and aseptic loosening 2.7%. HHS improved from 19.5 to 59.8. They did not find any statistically significant difference between two groups of CMACs (14). However, CTACs had cranialization and lateralization of implants without any impact on clinical outcomes. von Hertzberg-Boelch et al. followed 14 Monoflanged patients for average 3 years with 70% survival, 14% complications (PJI), 14% aseptic loosening, and 28.6% all cause revision rate. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score decreased from 64 to 30 at 1 year (31). Fröschen et al. studied 68 mixed mono and triflanged CMACs patients for 5 years with 77% implant survival, 36% revision, 34% complications (mostly PJI) and 2.9% aseptic loosening (32).
Various authors report encouraging midterm results using CTACs for massive acetabular bone loss. DeBoer et al. performed triflange component insertion in 18 patients (20 hips) with massive acetabular bone loss and pelvic discontinuity. At a mean follow-up of 10 years, none of the components had been revised for aseptic loosening, and healing of the discontinuity was radiographically evident in 18 of the 20 hips (90%) (25). We selected 7 studies from literature on CTAC. Berasi et al. followed 24 hips for 5 years (28). They reported 82% implant survival at 5 years. with 16% complication rate and 65 postoperative HHS for Paprosky Type 3B acetabular defect. Berasi et al. reported high rates of discontinuity healing and osseointegration of CTACs with host bone with no aseptic loosening at midterm follow-up. Winther et al. showed 94% implant survival rate with 5% complication rate and 81 postoperative HHS for their 39 revision THR for Pelvic discontinuity (29). Taunton et al. followed 57 CTAC revision THR patients with Paprosky type 3A/3B for minimum 5.5 years (27). They demonstrated 81% pelvic discontinuity healing with 30% all cause re-revision and among those, only 1.8% re-revision was linked to aseptic loosening of acetabular prosthesis. HHS was improved to 75. In a systematic review by De Martino et al. on survivorship of CTACs, they found all-cause revision free survivorship was 82.7% with an overall complication rate of 29% (dislocation and infection, 11% and 6.2% respectively) (45). Additionally, the incidence of aseptic loosening was 1.7%. Given the relatively recent application of CTACs, the average follow-up for these studies was only 4.7 years. More quality long-term studies are still needed to validate CTACs as a treatment option for massive bone loss.
Conclusions
There is no consensus regarding the best option for reconstructing massive acetabular defects. Thorough preoperative workup and planning is an absolute requirement for successful revision THR. While most of the moderate acetabular bone loss can be managed with cementless hemispherical acetabular shells with excellent long-term outcomes, reconstructing massive acetabular bone defects in revision THR remains a challenge. Depending on the size and location of the defect, various constructs have demonstrated long-term success as discussed in this review, but complications are not negligible. More recently, CTACs have been used to manage massive bone loss (with or without pelvic discontinuity) that may be otherwise difficult to achieve anatomic stability with other constructs. Although long-term data is sparse, the cost and complication rate is comparable to other reconstruction methods.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nemandra A Sandiford and Daniel Kendoff) for the series “Revision Total Hip Arthroplasty” published in Annals of Joint. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoj.amegroups.org/article/view/10.21037/aoj-23-23/rc
Peer Review File: Available at https://aoj.amegroups.org/article/view/10.21037/aoj-23-23/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.org/article/view/10.21037/aoj-23-23/coif). The series “Revision Total Hip Arthroplasty” was commissioned by the editorial office without any funding or sponsorship. A.F.K. reports that he receives IP royalties from Innomed; is a paid consultant for BodyCad, Zimmer, Ortho Development and United Ortho; is a board or committee member of AAOS and AAHKS; and has stock or stock options in Johnson & Johnson, Procter & Gamble and Zimmer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bayliss LE, Culliford D, Monk AP, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet 2017;389:1424-30. [Crossref] [PubMed]
- Retrieval Analysis of Contemporary Alternative Femoral Head Materials: Oxinium and Biolox Poster No. 2300. 55th Annual Meeting of the Orthopaedic Research Society. 2005;2:2300.
- Cnudde P, Nemes S, Bülow E, et al. Trends in hip replacements between 1999 and 2012 in Sweden. J Orthop Res 2018;36:432-42. [Crossref] [PubMed]
- Ledford CK, Perry KI, Hanssen AD, et al. What Are the Contemporary Etiologies for Revision Surgery and Revision After Primary, Noncemented Total Hip Arthroplasty? J Am Acad Orthop Surg 2019;27:933-8. [Crossref] [PubMed]
- Sculco PK, Wright T, Malahias MA, et al. The Diagnosis and Treatment of Acetabular Bone Loss in Revision Hip Arthroplasty: An International Consensus Symposium. HSS J 2022;18:8-41. [Crossref] [PubMed]
- Parvizi J, Tan TL, Goswami K, et al. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty 2018;33:1309-1314.e2. [Crossref] [PubMed]
- McNally M, Sousa R, Wouthuyzen-Bakker M, et al. The EBJIS definition of periprosthetic joint infection. Bone Joint J 2021;103-B:18-25. [Crossref] [PubMed]
- D'Antonio JA, Capello WN, Borden LS, et al. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res 1989;126-37. [Crossref] [PubMed]
- Ghanem M, Zajonz D, Heyde CE, et al. Acetabular defect classification and management: Revision arthroplasty of the acetabular cup based on 3-point fixation. Orthopade 2020;49:432-42. [Crossref] [PubMed]
- Gross AE. Revision arthroplasty of the acetabulum with restoration of bone stock. Clin Orthop Relat Res 1999;198-207. [Crossref] [PubMed]
- Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty 1994;9:33-44. [Crossref] [PubMed]
- Sporer SM. How to do a revision total hip arthroplasty: revision of the acetabulum. J Bone Joint Surg Am 2011;93:1359-66. [Crossref] [PubMed]
- Wirtz DC, Jaenisch M, Osterhaus TA, et al. Acetabular defects in revision hip arthroplasty: a therapy-oriented classification. Arch Orthop Trauma Surg 2020;140:815-25. [Crossref] [PubMed]
- Walter SG, Thomas TS, Kenndoff D, et al. Mid-term follow-up after all-size acetabular revision and proposal for a stability classification system. Hip Int 2020;30:431-7. [Crossref] [PubMed]
- Paxton ES Jr, Keeney JA, Maloney WJ, et al. Large acetabular defects can be managed with cementless revision components. Clin Orthop Relat Res 2011;469:483-93. [Crossref] [PubMed]
- Zhang J, Huang Y, Zhou B, et al. Mid-Term Follow-Up of Acetabular Revision Arthroplasty Using Jumbo Cups. Orthop Surg 2019;11:811-8. [Crossref] [PubMed]
- van Egmond N, De Kam DC, Gardeniers JW, et al. Revisions of extensive acetabular defects with impaction grafting and a cement cup. Clin Orthop Relat Res 2011;469:562-73. [Crossref] [PubMed]
- Schreurs BW, Slooff TJ, Buma P, et al. Acetabular reconstruction with impacted morsellised cancellous bone graft and cement. A 10- to 15-year follow-up of 60 revision arthroplasties. J Bone Joint Surg Br 1998;80:391-5. [Crossref] [PubMed]
- Huang Y, Tang H, Zhou Y, et al. Extended Ischiopubic Fixation Using Porous Metal Augments in Cementless Acetabular Reconstruction during Revision Total Hip Arthroplasty. Orthop Surg 2022;14:2480-8. [Crossref] [PubMed]
- Kosashvili Y, Backstein D, Safir O, et al. Acetabular revision using an anti-protrusion (ilio-ischial) cage and trabecular metal acetabular component for severe acetabular bone loss associated with pelvic discontinuity. J Bone Joint Surg Br 2009;91:870-6. [Crossref] [PubMed]
- Amenabar T, Rahman WA, Hetaimish BM, et al. Promising Mid-term Results With a Cup-cage Construct for Large Acetabular Defects and Pelvic Discontinuity. Clin Orthop Relat Res 2016;474:408-14. [Crossref] [PubMed]
- Durand-Hill M, Henckel J, Di Laura A, et al. Can custom 3D printed implants successfully reconstruct massive acetabular defects? A 3D-CT assessment. J Orthop Res 2020;38:2640-8. [Crossref] [PubMed]
- Zhang Y, Gao Z, Zhang B, et al. The application of custom-made 3D-printed titanium augments designed through surgical simulation for severe bone defects in complex revision total hip arthroplasty. J Orthop Traumatol 2022;23:37. [Crossref] [PubMed]
- Sporer SM, Bottros JJ, Hulst JB, et al. Acetabular distraction: an alternative for severe defects with chronic pelvic discontinuity? Clin Orthop Relat Res 2012;470:3156-63. [Crossref] [PubMed]
- DeBoer DK, Christie MJ, Brinson MF, et al. Revision total hip arthroplasty for pelvic discontinuity. J Bone Joint Surg Am 2007;89:835-40. [Crossref] [PubMed]
- Scharff-Baauw M, Van Hooff ML, Van Hellemondt GG, et al. Good results at 2-year follow-up of a custom-made triflange acetabular component for large acetabular defects and pelvic discontinuity: a prospective case series of 50 hips. Acta Orthop 2021;92:297-303. [Crossref] [PubMed]
- Taunton MJ, Fehring TK, Edwards P, et al. Pelvic discontinuity treated with custom triflange component: a reliable option. Clin Orthop Relat Res 2012;470:428-34. [Crossref] [PubMed]
- Berasi CC 4th, Berend KR, Adams JB, et al. Are custom triflange acetabular components effective for reconstruction of catastrophic bone loss? Clin Orthop Relat Res 2015;473:528-35. [Crossref] [PubMed]
- Winther SS, Petersen M, Yilmaz M, et al. Custom-made triflanged implants in reconstruction of severe acetabular bone loss with pelvic discontinuity after total hip arthroplasty consecutive cohort study: two to 11 years of follow-up. Bone Jt Open 2022;3:867-76. [Crossref] [PubMed]
- Walter SG, Randau TM, Gravius N, et al. Monoflanged Custom-Made Acetabular Components Promote Biomechanical Restoration of Severe Acetabular Bone Defects by Metallic Defect Reconstruction. J Arthroplasty 2020;35:831-5. [Crossref] [PubMed]
- von Hertzberg-Boelch SP, Wagenbrenner M, Arnholdt J, et al. Custom Made Monoflange Acetabular Components for the Treatment of Paprosky Type III Defects. J Pers Med 2021;11:283. [Crossref] [PubMed]
- Fröschen FS, Randau TM, Hischebeth GTR, et al. Mid-term results after revision total hip arthroplasty with custom-made acetabular implants in patients with Paprosky III acetabular bone loss. Arch Orthop Trauma Surg 2020;140:263-73. [Crossref] [PubMed]
- Deirmengian GK, Zmistowski B, O'Neil JT, et al. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am 2011;93:1842-52. [Crossref] [PubMed]
- Gaffey JL, Callaghan JJ, Pedersen DR, et al. Cementless acetabular fixation at fifteen years. A comparison with the same surgeon's results following acetabular fixation with cement. J Bone Joint Surg Am 2004;86:257-61. [Crossref] [PubMed]
- Della Valle CJ, Shuaipaj T, Berger RA, et al. Revision of the acetabular component without cement after total hip arthroplasty. A concise follow-up, at fifteen to nineteen years, of a previous report. J Bone Joint Surg Am 2005;87:1795-800. [Crossref] [PubMed]
- Park DK, Della Valle CJ, Quigley L, et al. Revision of the acetabular component without cement. A concise follow-up, at twenty to twenty-four years, of a previous report. J Bone Joint Surg Am 2009;91:350-5. [Crossref] [PubMed]
- von Roth P, Abdel MP, Harmsen WS, et al. Uncemented jumbo cups for revision total hip arthroplasty: a concise follow-up, at a mean of twenty years, of a previous report. J Bone Joint Surg Am 2015;97:284-7. [Crossref] [PubMed]
- Nwankwo CD, Ries MD. Do jumbo cups cause hip center elevation in revision THA? A radiographic evaluation. Clin Orthop Relat Res 2014;472:2793-8. [Crossref] [PubMed]
- Busch VJ, Gardeniers JW, Verdonschot N, et al. Acetabular reconstruction with impaction bone-grafting and a cemented cup in patients younger than fifty years old: a concise follow-up, at twenty to twenty-eight years, of a previous report. J Bone Joint Surg Am 2011;93:367-71. [Crossref] [PubMed]
- Verspeek J, Nijenhuis TA, Kuijpers MFL, et al. What Are the Long-term Results of Cemented Revision THA with Use of Both Acetabular and Femoral Impaction Bone Grafting in Patients Younger Than 55 Years? Clin Orthop Relat Res 2021;479:84-91. [Crossref] [PubMed]
- Lee PT, Raz G, Safir OA, et al. Long-term results for minor column allografts in revision hip arthroplasty. Clin Orthop Relat Res 2010;468:3295-303. [Crossref] [PubMed]
- Goodman S, Saastamoinen H, Shasha N, et al. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty 2004;19:436-46. [Crossref] [PubMed]
- Hasenauer MD, Paprosky WG, Sheth NP. Treatment options for chronic pelvic discontinuity. J Clin Orthop Trauma 2018;9:58-62. [Crossref] [PubMed]
- Fröschen FS, Randau TM, Walter SG, et al. Use of custom-made acetabular components (CMAC) as part of a two-stage procedure in patients with severe periacetabular bone loss. Oper Orthop Traumatol. 2022;34:361-71. [PubMed]
- De Martino I, Strigelli V, Cacciola G, et al. Survivorship and Clinical Outcomes of Custom Triflange Acetabular Components in Revision Total Hip Arthroplasty: A Systematic Review. J Arthroplasty 2019;34:2511-8. [Crossref] [PubMed]
Cite this article as: Pandey AK, Zuke WA, Surace P, Kamath AF. Management of acetabular bone loss in revision total hip replacement: a narrative literature review. Ann Joint 2024;9:21.


