
Trabecular titanium cups in hip revision surgery: a systematic review of the literature
Highlight box
Key findings
• Trabecular titanium cups provide optimal clinical outcomes in hip revision surgery in the presence of an acetabular bone defect.
What is known and what is new?
• Revision surgery in the presence of severe acetabular bone defects represents one of the most complex challenges for hip surgeons. This study reviewed the current literature on using Delta Trabecular Titanium (TT) revision cups in cases of severe acetabular bone defects.
• This is the first systematic review that analyzes the outcomes of using trabecular titanium cups in hip revision surgery.
What is the implication, and what should change now?
• The use of Delta TT revision cups has resulted in optimal clinical outcomes with acceptable complication rates and good patient reported outcomes measures (PROMs). Despite this, postoperative dislocation remains the main problem. Furthermore, the aseptic loosening rate reported with this specific implant is in line with or even lower than that reported by similar implants.
Introduction
Total hip arthroplasty (THA) has long been considered the gold standard for end-stage hip osteoarthritis as it offers optimal clinical outcomes and long-term survival, as documented by published data in long-term outcome studies and registries (1-3). In recent decades, the number of surgeries performed annually has been steadily increasing, causing an increase in the number of revision procedures (4,5). It is estimated that 35,000 people undergo revision THA surgery each year in the United States, which is expected to increase by 137% by 2030 (6).
Revision surgery in the presence of severe acetabular bone defects represents one of the most complex challenges for hip surgeons (7,8). The primary goal of hip revision surgery is to obtain a stable acetabular component and restore joint biomechanics (center of rotation and femoral offset) to prevent early migration, loosening, and instability (9,10). There are numerous alternative methods to perform acetabular revision depending on the location and extent of bone deficit, including cementless hemispheric cups, structural allograft, antiprotuse cages, jumbo cups, trabecular metal cups, and custom-made triflange implants (11-16). The Delta Trabecular Titanium (TT) revision system (Lima Corporate, Udine, Italy) outer surface is characterized by a highly porous area similar to the cancellous bone. The diameter porosity should stimulate implant osteoblast adhesion, promoting cup osteointegration with natural bone and early and strong bone growth (17,18). This study reviewed the current literature on using Delta TT revision cups.
As primary outcomes in this systematic review, the (I) overall survival, (II) prevalence of postoperative aseptic loosening of the acetabular component, (III) and patient-reported outcomes measures (PROMs) of patients undergoing hip revision surgery using delta TT cups were examined. As secondary outcomes, (IV) the reason for other complications, (V) the prevalence of the use of modular components (hemispherical and internal modules), and the prevalence of the use of bone grafts were evaluated. We present this article in accordance with the PRISMA reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-28/rc).
Methods
Implant design
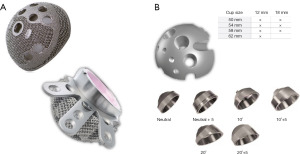
The Delta TT revision system, introduced into clinical practice in 2008, consists of two revision cups, the Delta One TT and the Delta Revision TT. Delta One TT is a hemispherical multi-hole acetabular shell made of 3D-printed titanium alloy (17,18) (Figure 1). Delta TT Revision is a cup-cage with a caudal hook and three cranial wings to achieve primary cup stability in severe defects. The outer surfaces of Delta TT One and Revision cups are characterized by a highly porous surface (with a porosity of about 65%) with an average pore diameter of 640 µm. Both cups accept connection with hemispherical modules that screws can attach in three different positions without using bone cement. Two sizes of hemispherical modules (HM) can be used from 50 mm cups according to the extent of the defect (12 and 18 mm). In addition, this system allows internal modules (IM) or “spacers” to increase the offset of the system and coverage. There are different internal modules: +5 mm, 10° coverage, +5 mm, and 10° coverage, 20° coverage, and 5 mm and 20° coverage. Finally, both cups allow dual mobility liners (Figure 1) (19-26).
Search strategy and criteria
This systematic review was performed according to the PRISMA criteria (26). A comprehensive manual search was performed on PubMed, Embase, Scopus, and the Cochrane Database of systematic reviews to identify relevant studies on the outcome of Delta TT cups in revision surgery in patients with a severe acetabular bone defect. The search covered all studies accessible from January 2008 to December 2022, using the following MeSH terms: [(hip arthroplast*) OR (hip replacement) OR (THR) OR (THA) OR (revision*) AND ((trabecular titanium) OR (TT) OR (Delta TT)]. Additional relevant studies were searched in the reference lists of the most pertinent articles. The study was registered in the International Prospective Register of Systematic Reviews (PROSPERO).
Inclusion and exclusion criteria
The list of articles was exported into EndNote X7 (Thomson Reuters). All duplicates were removed. Titles and abstracts were screened for possible relevant studies. Then, full-text articles of relevant studies were reviewed by applying inclusion and exclusion criteria. We included clinical studies published in English, reporting the clinical outcome of patients that underwent revision THA with Delta TT Cups. We excluded review articles, non-clinical studies, biomechanical reports, instructional course reports, case series and articles written in languages other than English. Two authors separately reviewed the studies (GC, FDM). A third author was consulted in case of disagreement (PC). The initial search generated a list of 386 records. A list of 18 studies was screened for eligibility by applying inclusion and exclusion criteria. Furthermore, a cross-reference check was performed to identify other relevant papers. Eight studies met the inclusion criteria and were included in the final analysis of this systematic review (Figure 2) (19-26).
Data collection and extraction
Two authors (GC and FDM) retrieved all information related to the studies included. The parameters evaluated were level of evidence (LoE), length of the follow-up period, number of patients initially included, patients deceased or lost to follow-up, mean age at the time of surgery and mean body mass index (BMI) at the time of surgery, classification of the bone defect according to Paprosky’s criteria (27,28), implant characteristics such as type of cup used, HM used, IM used, and bone allograft used. Finally, the postoperative outcomes, such as complications, reinterventions, and PROMs, were evaluated.
Primary and secondary outcomes
The primary objectives of this study were to evaluate the overall survival and free-of-aseptic-loosening survival of delta TT cups in hip revision surgery. Therefore, PROMs were assessed, and average preoperative and postoperative scores (at the last follow-up if more postoperative scores are reported) and standard deviation (SD) if present (or range) were collected. As secondary outcomes, information was collected on the reason for other complications and the use of modular components and bone grafts in hip revision with delta TT cups.
Assessment of study quality
The Methodological Index for Non-Randomized Studies Criteria (MINORS) score was used to assess the quality of the included studies. The MINORS score was frequently applied in the literature about systematic reviews on arthroplasty studies and is helpful in evaluating the quality of non-randomized surgical research (29). MINORS score consists of eight questions with a score range from 0 to 2. The “item” was scored 0 if it is not provided in the study, it was scored 1 if it is partially described, or it is scored 2 if it is well described. A study was graded as “good” quality if the score was between 11 and 16 points, “moderate” if the score was between 6 and 10, or “poor” if the score was lower than 5. Two authors (GC and FG) separately calculated the MINORS score, and in case of disagreement, a third author (FB) was consulted, and the final decision was reached. The average MINORS score was 10 (9 to 11).
Statistical analysis
Frequency and percentages are used to present categorical variables. Continuous variables were presented as means values and standard deviation or range. Statistical significance was defined as a P value <0.05.
Results
Demographic characteristics and surgical data
The eight studies initially included 523 hips. After excluding patients who died or lost to follow-up, the final number of hips included in the analysis was 477. The average age at the time of surgery was 71.8 years (67.4 to 79 years). There were 253 men (41.2%) and 241 women (58.8%). The average follow-up length was 56.1 months (20.8 to 91 months). The general characteristics of the included studies are reported in Table 1. Three studies included patients that underwent surgery with only Delta TT revision cups for severe acetabular defects (20,21,24). In comparison, the other five studies reported results of both Delta TT one and Delta TT revision cups in moderate and severe revision (19,22,23,25,26). Delta TT Cup was used in 3.9% of cases (19 hips), Delta TT One Cup was used in 228 hips (48.8%), and Delta TT Revision was used in 240 cases (49.3%). Aseptic loosening was the most frequent reason for revision (320 cases, 70.6%), followed by hip instability (61 patients, 13.5%) and periprosthetic joint infection (48 cases, 10.6%). Acetabular bone defects were classified according to Paprosky criteria (Table 2) (27,28). Type IIIa was the most frequent acetabular bone defect (130 cases, 26.4%), followed by Type IIIb (104 cases, 21.1%) and Type IIb (108 cases, 21.9%).
Table 1
| Study | LoE | No. of hips initial/final | Patients died or lost to follow-up, N (%) |
Cup used [No.] | Mean follow-up (months), [SD] or (range) | Mean age (years), [SD] or range | Male/female | Mean BMI (kg/m2), [SD] or range | MINORS score |
|---|---|---|---|---|---|---|---|---|---|
| Cozzi Lepri [2022] (19) | IV | 107/85 | 22 (20.6) | Delta TT One [30], Delta TT Revision [55] | 73.4 (24 to 122.4) | 67.8 (32 to 83) | 50/35 | 26.9 (25.4 to 27.7) | 9 |
| El Ghazawy [2022] (20) | IV | 24/24 | 0 (0.0) | Delta TT Revision [24] | 20.8 (14 to 30) | 56 (30 to 67) | 18/6 | N/A | 10 |
| Jara-García [2023] (21) | IV | 37/35 | 2 (5.4) | Delta TT Revision [37] | 61 (14 to 117) | 67.4 (43 to 89) | 10/24 | 27.2 [5.7] | 9 |
| Perticarini [2021] (22) | IV | 104/95 | 9 (8.7) | Delta TT One [56], TT Revision [39] | 91 (24 to 146) | 79 (29 to 90) | 69/35 | 25.7 [3.7] | 10 |
| De Meo [2018] (23) | IV | 64/58 | 6 (11.1) | Delta TT One [39], TT Revision [25] | 48.3 (38 to 82.3) | 78.4 (42 to 87) | 27/37 | 26.1 (23.1 to 33.2) | 11 |
| Munegato [2018] (24) | IV | 37/36 | 1 (2.7) | Delta TT Revision [37] | 39.8 (12 to 91.5) | 75 (45 to 92) | 12/22 | N/A | 10 |
| Gallart [2016] (25) | IV | 69/64 | 5 (7.2) | Delta TT One [54], Delta TT Revision [19] | 30.5 [16.9] | 70.7 [10.3] | 37/32 | N/A | 10 |
| Steno [2015] (26) | IV | 81/80 | 1 (1.2) | Delta TT [19], TT One [49], TT Revision [13] | 38.1 (24 to 62) | 68.3 (32 to 84) | 30/50 | N/A | 11 |
| Overall | IV | 523/477 | 46 (8.8) | Delta TT [19], TT One [228], TT Revision [240] | 56.1 (20.8 to 91) | 71.8 (67.4 to 79) | 253/241 | 26.5 (25.7 to 27.2) | Mean 10 (range, 9 to 11) |
LoE, level of evidence; SD, standard deviation; BMI, body mass index; MINORS, Methodological Index for Non-Randomized Studies; TT, trabecular titanium; N/A, not available.
Table 2
| Study | Type I | Type IIa | Type IIb | Type IIc | Type IIIa | Type IIIb | Type IV |
|---|---|---|---|---|---|---|---|
| Cozzi Lepri [2022] (19) | 0 | 0 | 23 | 20 | 24 | 18 | 0 |
| El Ghazawy [2022] (20) | 0 | 0 | 2 | 0 | 7 | 15 | 0 |
| Jara-García [2023] (21) | 0 | 0 | 0 | 0 | 20 | 17 | 0 |
| Perticarini [2021] (22) | 0 | 23 | 17 | 13 | 22 | 19 | 0 |
| De Meo [2018] (23) | 0 | 0 | 25 | 15 | 15 | 9 | 0 |
| Munegato [2018] (24) | 0 | 0 | 5 | 7 | 15 | 9 | 0 |
| Gallart [2016] (25) | 19 | 12 | 9 | 16 | 12 | 4 | 0 |
| Steno [2015] (26) | 9 | 11 | 27 | 6 | 15 | 13 | 0 |
| Overall | 28 (5.7) | 46 (9.3) | 108 (21.9) | 77 (15.6) | 130 (26.4) | 104 (21.1) | 0 |
Data are represented as number or n (%).
Overall survivorship and aseptic loosening
The all-cause survivorship, considering all cup revision as a failure, reported in this systematic review was 92.5% (441 out of 477 cases) at an average follow-up of 56.1 months. The most frequent cause of revision of the TT cups was septic loosening in 14 cases (2.9%), cup revision due to aseptic loosening in seven patients (1.5%), and due to recurrent dislocation in six cases (1.3%) (Table 3).
Table 3
| Study | No hips final | Dislocation | Infections | Aseptic loosening | Periprosthetic fracture | Survivorship | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Surgical | Overall | Surgical | Overall | Surgical | Overall | Surgical | All reoperations | Cups revision | Aseptic loosening | ||||||
| Cozzi Lepri [2022] (19) | 85 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 97.6% | 97.6% | 98.8% | ||||
| El Ghazawy [2022] (20) | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100% | 100% | 100% | ||||
| Jara-García [2023] (21) | 35 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 91.9% | 94.3% | 94.6% | ||||
| Perticarini [2021] (22) | 95 | 7 | 5 | 7 | 7 | 1 | 1 | 2 | 2 | 84.2% | 88.4% | 98.8% | ||||
| De Meo [2018] (23) | 58 | 3 | 3 | 2 | 2 | 1 | 1 | 0 | 0 | 89.7% | 91.4% | 98.3% | ||||
| Munegato [2018] (24) | 36 | 3 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 88.9% | 97.2% | 100% | ||||
| Gallart [2016] (25) | 64 | 2 | 2 | 3 | 3 | 2 | 2 | 0 | 0 | 89.1% | 89.1% | 96.9% | ||||
| Steno [2015] (26) | 80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100% | 100% | 100% | ||||
| Overall | 477 | 16 (3.4) | 14 (2.9) | 14 (2.9) | 14 (2.9) | 7 (1.5) | 7 (1.5) | 2 (0.4) | 2 (0.4) | 441 (92.5) | 28 (5.9) | 470 (98.5) | ||||
Data are represented as number, percent, or n (%).
Prevalence of aseptic loosening
The prevalence of aseptic loosening was 1.5% (7 cases out of 477 patients); reoperation was necessary in all cases. The cup revision was performed in four cases with the same Delta TT cup. In one of them, a hemispherical module was added to increase the primary stability of the implant. In one case, the loosening was associated with the graft’s reabsorption, and a cage and a cemented cup were necessary. In two cases, the type of reoperation was not specified (22). Three studies reported no reoperation due to aseptic loosening in their case series (20,24,26).
PROMS
Harris Hip Score (HHS): five studies reported the preoperative and postoperative average HHS (213 patients) (19-22,24). The average preoperative score was 42.5 (30 to 54.7) to an average postoperative score of 86.7 (83.7 to 89.7). The average score improved from a mean of 44.2 points between the preoperative and postoperative periods. All five studies reported that the postoperative scores were graded as “good” (between 80 and 90 points).
Merle D’Aubigne Hip Score: Three studies reported the preoperative and postoperative average Merle d’Aubigné Hip Score (179 patients) (21,25,26). The average preoperative score was 7.2 (4.7 to 11.1) to an average postoperative score of 13.2 (9.1 to 17.1).
Reason for other complications
The overall complication rate in this systematic review was 8.7% (36 out of 477 cases). The most frequent complication was hip dislocation, with a prevalence of 3.4% (16 cases), followed by periprosthetic joint infection in 2.9% (14 patients), aseptic loosening in 1.5% (7 cases), and periprosthetic fractures at 0.5% (2 patients). The prevalence of dislocation in the present study was 3.4% (16 cases out of 477), and in 14 cases of them, a reoperation was necessary (2.9%). Internal module spacer exchange was the most frequent reoperation to treat recurrent dislocation in 8 cases (1.7%), followed by cup revision in 6 patients (1.3%). A closed reduction was sufficient in three cases (0.6%). The prevalence of Periprosthetic joint infection was 2.9% (14 cases out of 477), and reoperation was necessary in all cases. Two-stage revision with the use of an interim spacer was performed in 13 cases (2.7%). A DAIR procedure was performed in one case (the patient refused the two-stage revision).
Use of modular components and bone graft
Six out of eight studies (395 hips) reported the use of IM or “spacers” (Table 4) (19,20,22-24,26). The prevalence of the use of spacers was 42.7% (169 hips out of 395). In 131 cases, the spacers’ type was specified, while in 38 cases it was not. The most frequently used spacer was the “20°” in 31.4% of cases (53 hips).
Table 4
| Characteristics | No. | % |
|---|---|---|
| Type of cup | 487 | 100 |
| Delta TT | 19 | 3.9 |
| Delta TT One | 228 | 46.8 |
| Delta TT Revision | 240 | 49.3 |
| Bone graft | 336 | 70.4 |
| Morselized bone graft | 307 | 91.4 |
| Bulk allograft | 18 | 5.3 |
| Synthetic bone | 11 | 3.3 |
| Spacers* | 169 | 42.7 |
| 5 mm | 19 | 11.2 |
| 10° | 32 | 18.9 |
| 5 mm + 10° | 11 | 6.5 |
| 20° | 53 | 31.4 |
| 5 mm + 20° | 16 | 9.5 |
| Not specified | 38 | 22.5 |
| Hemispherical augment** | 86 | 18.8 |
| 12 mm | 30 | 34.9 |
| 18 mm | 17 | 19.8 |
| Not specified | 39 | 45.3 |
*, percentage on “Spacers” are calculated on 395 hips, no data were provided by the studies of Jara-García and Gallart. **, percentage on “Hemispherical augment” are calculated on 458 hips, data were provided by the study of Jara-García (21). TT, trabecular titanium.
Seven out of eight studies reported HM use or “augment” (458 hips) (19,20,22-26). The prevalence of the use of hemispherical modules was 18.8% (86 hips). In 47 cases, the HM size was specified, while in 39 cases it was not. The most frequent size implanted was 12 mm (30 cases, 34.9%).
Bone graft: All studies reported data about the prevalence and type of bone graft used in hip revision surgery (19,25-43). Bone graft was frequently used in association with TT cups in revision for acetabular bone defects. The prevalence reported in our study was 70.4% (336 hips, Table 4). Morselized bone graft was the most frequent type of graft used (397 hips, 91.4% of cases), followed by a bulk allograft from the femoral head (18 hips, 5.3% of cases), while the synthetic bone was used in 3.3% of cases (11 hips).
Discussion
The number of THA performed yearly is constantly increasing; consequently, revision procedures are rising (5,6). Acetabular revision in the presence of a severe bone defect represents one of the most complex challenges (11,16-31). In the last decades, different reconstruction techniques using other implants and components have been used (11,16-33). To our knowledge, no reviews were performed on the acetabular revision outcome with TT Cups. We identified eight relevant studies that describe the outcomes of cup reconstruction using the Delta TT cups family (19-26). Our systematic review noted that the all-cause survivorship rates, aseptic loosening survivorship rate, and PROMs were favorable and in line with the outcomes reported with similar implants (11,16). In addition, we reported that both the modular components, HM and IM, were used frequently during revision surgery in association with bone allograft.
This systematic review has several strengths. It is the first study that analyzes the TT cups outcomes in hip revision surgery. Second, almost all studies had medium-term follow-up demonstrating how the results achieved during the hip revisions were maintained over time.
There are several limitations to the present systematic review. First, the original studies’ quality, the variety of inclusion and exclusion criteria and reporting procedures, and the number of patients studied. Second, in addition to having various patient populations, different surgical indications, surgeon expertise, study designs, and follow-up rates were included. Third, unduly optimistic estimates of survivability rates and PROMs may have been caused by selection, transfer, and evaluation biases. Fourth, the included studies used various PROMs, and average values and SDs were presented inconsistently, which further restricted the number of comparative research. Four studies (213 hips) reported the average preoperative and postoperative HHS (19,20,22,24), while three studies (170 hips) reported the average preoperative and postoperative Mele D’Aubigne Score (21,25,26). Lastly, there was variability in the postoperative care in different studies following acetabular reconstruction with TT cups.
All-cause survivorship for TT cups was 92.5%, with two studies reporting overall survivorship of 100% at an average follow-up of 20.8 and 38.1 months, respectively (20,26). Two studies reported a cups survivorship inferior of 90%, Perticarini et al. (22) reported a cups survivorship of 88.4% at an average follow-up of 7.6 years, and Gallart et al. (25) reported a survivorship of 89.1% at an average follow-up of 2.5 years. The other studies reported a survivorship superior of 90% (19,21,23,24). The all-cause survivorship reported in this review aligns with the survivorship rate reported in a recent systematic review of different implant types in hip reconstruction in the presence of severe acetabular bone defects (11,16). Shen et al. reported an overall cup failure of 6.8% using trabecular metal (16). Similar cup failure was reported by Malahias et al. (13) in a cohort of patients that underwent hip revision surgery using trabecular metal augments. The authors described reported a failure rate of 5.7% in an analysis of 769 hip revisions included in 15 studies. The survival rate of TT cups was in line with the survival rate of uncemented Jumbo cups. In a recent systematic literature review, Wang et al. (15) reported an overall reoperation rate of 8.6% with a cup survivorship of 95%. The higher survivorship reported with the Jumbo cup could be explained due to the less severe acetabular bone deficiency of patients included in their study. Aprato et al. (12) performed a systematic review on using cages in acetabular revision for severe bone defects (28 articles, 1,327 hips) with an average follow-up of 8.8 years, reporting that the overall revision rate was 6.3%. If we compare the overall survivorship of TT cups with the overall survivorship of custom triflange, we described that the latter had a higher reoperation rate, as reported by De Martino et al. (11) in a systematic review of 17 studies on 579 hips reported a survivorship of 82.7%. Comparing the outcome of a different implant is not easy, mainly due to the various bone defect severity of the groups of patients.
Considering aseptic loosening as a failure, we observed an overall survivorship of Delta TT Cups of 98.5% (6 aseptic loosening of 392 revisions). The results reported in our review are slightly better than those reported with similar implants in cases with severe acetabular bone defects (11,16). Aseptic loosening with TT cups aligns with the prevalence reported using TM cups (16). In a recent systematic review of the use of TM cups for revision in complex acetabular reconstruction, Shen et al. described a failure rate due to aseptic loosening of 1.8% (ranging from 1.1 to 7.9 according to the different included studies) (16). Similar data were also reported in a systematic review of the outcome of cages in acetabular reconstruction, in which Aprato et al. reported a revision rate for aseptic loosening of 6% (12). A similar revision rate was also reported by De Martino et al. (11) with the use of custom triflange for revision in the presence of severe bone defect (prevalence of 1.7%). The low rate of aseptic loosening reported in our review with the use of these cups could be explained thanks to the osteointegration and osteoinductive biomechanical properties of the TT (16,17). TT cups are produced by electron beam melting (EBM) (16,17). This technology allows the creation of a compound characterized by a porosity similar to the cancellous bone. The diameter porosity of 650 µm should stimulate the osteoblast adhesion to the implants, increasing the cups’ osseointegration with the natural bone, resulting in an early and strong bone ingrowth (16,17).
Another critical concern in acetabular reconstruction in severe acetabular defects is to restore the biomechanical parameters such as femoral offset and hip center of rotation to reduce the risk of postoperative dislocation (35,36). Often these patients had a concomitance of abductor muscle weakness or absence due to the multiple previous surgical procedures that have been subjected to various revision surgeries. Soft tissue laxities associated with a severe acetabular bone defect make hip reconstruction in this group of patients at high risk for postoperative dislocation (36,37). Sometimes achieving primary stability of the implant does not allow for obtaining adequate coverage [40°±10° of lateral coverage and 25°±10° of anteversion according to the Lewinnek Safe Zone (38,39)]. Delta TT cups allow inserting a modular internal module or “spacer” to increase the femoral offset, consequently increasing the soft tissue tensioning and the acetabular coverage up to 20°. In our systematic review, dislocation was the most frequent postoperative complication, with a prevalence of 4.1% (16 cases out of 392). In 14 out of 16 cases, a further surgical procedure was necessary. A cup revision was performed in only six patients (1.5%), while a modular component change was required in the remaining eight cases (2%). Theoretically, using these modular cups reduces the risk of dislocation after surgery, allowing to achieve a less invasive surgery in case of postoperative recurrent dislocation (40,41). The prevalence of use of a non-neutral spacer in our review was 45.2% [all studies reported the use of internal modules to expect for Jara-García et al. (21) and Gallart et al. (25)], with the 20° coverage spacer as the most frequent implanted modules in 16.1% of cases (Table 4). Jara-García et al. (21) reported a single post-traumatic dislocation in their series that was managed with “spacer” exchange despite the cups being positioned in a suboptimal position (6° of anteversion), a neutral spacer was changed with a + 5 mm offset and a + 20° anterior coverage spacer. The patient didn’t report any postoperative dislocation after reoperation. Perticarini et al. (22) reported a higher dislocation rate (7.4%). Three cases were managed nonoperatively with closed reduction, two cases were treated with internal module changes, and cup revision was necessary in three patients. All three cases of recurrent dislocation reported by De Meo et al. (23) were treated with spacer exchange.
Although several studies have considered using bone grafts in cases of inadequate acetabular bone stock, a generally agreed-upon position has yet to be established. Consequently, a surgeon’s decision is frequently influenced by personal preference, and many different techniques have been suggested. In the present study, the prevalence of bone graft was 53.4%, while hemispherical modules were used in 20.1% of cases. The high prevalence of both bone graft and hemispherical modules could be explained due to the severity of bone defects. In the present study, acetabular bone defects type IIIa (severe superolateral migration greater than 2 cm) were the most frequent, followed by type IIIb defects (severe superomedial migration greater than 2 cm). with a prevalence of 26% and 21.1%. El Ghazawy et al. (20) suggest a partial weight bearing in the postoperative period to protect the structural bone graft, while Jara-García et al. (21) prohibit weight-bearing for one month after revision surgery, followed by partial weight bearing until 12 weeks postoperatively. Munegato et al. (24) was the only study that classified bone graft incorporation. They reported full incorporation of the graft into the native bone in 60% of cases, an integration between the native bone and the graft in 34.3% and reabsorption or lack of integration in 5.7%. Two authors reported the reabsorption of the bone allograft directly caused a case of aseptic loosen-ingraft. Perticarini et al. (22) reported a revision due to a loosened cup one year after the revision, managed with a cage and a cemented cup. Gallart et al. (25) used morselized bone grafts in fifteen cases and performed a revision for aseptic loosening of the cup in a single patient due to bone chip reabsorption.
Good clinical outcomes and PROMs with acceptable complication rates characterize the Delta TT revision cups. They may be applied to treat different acetabular bone defects due to their intraoperative modularity, biomechanical osteointegration, and osteoinductive properties. Moreover, the aseptic loosening rate reported with this specific implant is in line with or even lower than that reported by similar implants. Despite this, postoperative dislocation remains the main problem.
Conclusions
Managing severe acetabular bone defects remains one of the most complex challenges for hip reconstructive surgeons. Several different surgical techniques were described, and many implants were produced. Since the introduction of TT cups in 2008, many authors have reported optimal clinical outcomes with an acceptable complication rate and good PROMs. Despite the wide range of acetabular bone defects that could be filled with modular components, postoperative dislocation remains the main issue. On the other hand, the prevalence of aseptic loosening reporting with this specific implant is in line with or inferior to the rate reported by similar implants.
Acknowledgments
The authors extend profound gratitude to Dr. Pietro Cavaliere and Professor Alessandro Massè, who served as research supervisors, for the patient guidance, enthusiastic encouragement, and valuable critiques provided throughout the course of this research endeavor.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giuseppe Solarino and Giuseppe Marongiu) for the series “Modular Implants for Revision Arthroplasty in Orthopedics” published in Annals of Joint. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://aoj.amegroups.org/article/view/10.21037/aoj-23-28/rc
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-28/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-28/coif). The series “Modular Implants for Revision Arthroplasty in Orthopedics” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Park JW, Kim HS, Kim KC, et al. A 10- to 12-year follow-up study of delta ceramic-on-ceramic total hip arthroplasty. Arch Orthop Trauma Surg 2023;143:5385-94. [Crossref] [PubMed]
- Mancino F, Tornberg H, Jones CW, et al. The exeter cemented stem provides outstanding long-term fixation and bone load at 15 years follow-up: A systematic review and meta-analysis. J Orthop Surg (Hong Kong) 2023;31:10225536231153232. [Crossref] [PubMed]
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007;370:1508-19. [Crossref] [PubMed]
- Matsuoka H, Nanmo H, Nojiri S, et al. Projected numbers of knee and hip arthroplasties up to the year 2030 in Japan. J Orthop Sci 2023;28:161-6. [Crossref] [PubMed]
- Rasmussen MB, El-Galaly A, Daugberg L, et al. Projection of primary and revision hip arthroplasty surgery in Denmark from 2020 to 2050. Acta Orthop 2022;93:849-53. [Crossref] [PubMed]
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780-5. [Crossref] [PubMed]
- Mancino F, Cacciola G, Di Matteo V, et al. Reconstruction options and outcomes for acetabular bone loss in revision hip arthroplasty. Orthop Rev (Pavia) 2020;12:8655. [Crossref] [PubMed]
- Mancino F, Di Matteo V, Mocini F, et al. Survivorship and clinical outcomes of proximal femoral replacement in non-neoplastic primary and revision total hip arthroplasty: a systematic review. BMC Musculoskelet Disord 2021;22:933. [Crossref] [PubMed]
- Kaiser D, Ried E, Zingg PO, et al. Acetabular reconstruction with femoral head autograft in primary total hip arthroplasty through a direct anterior approach is a reliable option for patients with secondary osteoarthritis due to developmental dysplasia of the hip. Arch Orthop Trauma Surg 2022;142:2957-64. [Crossref] [PubMed]
- Warschawski Y, Morgan S, Garceau SP, et al. Does Revision of an Acetabular Shell With Insertion of an Uncemented Constrained Liner Confer Benefit Over Cementing Into a Well-Ingrown Shell? J Arthroplasty 2022;37:1631-5. [Crossref] [PubMed]
- De Martino I, Strigelli V, Cacciola G, et al. Survivorship and Clinical Outcomes of Custom Triflange Acetabular Components in Revision Total Hip Arthroplasty: A Systematic Review. J Arthroplasty 2019;34:2511-8. [Crossref] [PubMed]
- Aprato A, Olivero M, Branca Vergano L, et al. Outcome of cages in revision arthroplasty of the acetabulum: a systematic review. Acta Biomed 2019;90:24-31. [PubMed]
- Malahias MA, Ma QL, Gu A, et al. Outcomes of Acetabular Reconstructions for the Management of Chronic Pelvic Discontinuity: A Systematic Review. J Arthroplasty 2020;35:1145-1153.e2. [Crossref] [PubMed]
- Jain S, Grogan RJ, Giannoudis PV. Options for managing severe acetabular bone loss in revision hip arthroplasty. A systematic review. Hip Int 2014;24:109-22. [Crossref] [PubMed]
- Wang Q, Wang Q, Liu P, et al. Clinical and radiological outcomes of jumbo cup in revision total hip arthroplasty: A systematic review. Front Surg 2022;9:929103. [Crossref] [PubMed]
- Shen X, Qin Y, Li Y, et al. Trabecular metal versus non-trabecular metal acetabular components for acetabular revision surgery: A systematic review and meta-analysis. Int J Surg 2022;100:106597. [Crossref] [PubMed]
- Diez-Escudero A, Carlsson E, Andersson B, et al. Trabecular Titanium for Orthopedic Applications: Balancing Antimicrobial with Osteoconductive Properties by Varying Silver Contents. ACS Appl Mater Interfaces 2022;14:41751-63. [Crossref] [PubMed]
- Brogini S, Sartori M, Giavaresi G, et al. Osseointegration of additive manufacturing Ti-6Al-4V and Co-Cr-Mo alloys, with and without surface functionalization with hydroxyapatite and type I collagen. J Mech Behav Biomed Mater 2021;115:104262. [Crossref] [PubMed]
- Cozzi Lepri A, Innocenti M, Galeotti A, et al. Trabecular titanium cups in acetabular revision arthroplasty: analysis of 10-year survivorship, restoration of center of rotation and osteointegration. Arch Orthop Trauma Surg 2022;142:3523-31. [Crossref] [PubMed]
- El Ghazawy AK, Bassiony AA, Abdelazim H, et al. Acetabular revision using trabecular titanium (Delta TT) revision cups: A retrospective case series. SICOT J 2022;8:49. [Crossref] [PubMed]
- Jara-García F, Diranzo-García J, Estrems-Díaz V, et al. Trabecular titanium implants in complex acetabular revision surgery. Rev Esp Cir Ortop Traumatol 2023;67:94-101. [Crossref] [PubMed]
- Perticarini L, Rossi SMP, Medetti M, et al. Clinical and radiological outcomes of acetabular revision surgery with trabecular titanium cups in Paprosky type II and III bone defects. J Orthop Traumatol 2021;22:9. [Crossref] [PubMed]
- De Meo F, Cacciola G, Bellotti V, et al. Trabecular Titanium acetabular cups in hip revision surgery: mid-term clinical and radiological outcomes. Hip Int 2018;28:61-5. [Crossref] [PubMed]
- Munegato D, Bigoni M, Sotiri R, et al. Clinical and radiological outcomes of acetabular revision with the Delta Revision TT cup. Hip Int 2018;28:54-60. [Crossref] [PubMed]
- Gallart X, Fernández-Valencia JA, Riba J, et al. Trabecular TitaniumTM cups and augments in revision total hip arthroplasty: clinical results, radiology and survival outcomes. Hip Int 2016;26:486-91. [Crossref] [PubMed]
- Steno B, Kokavec M, Necas L. Acetabular revision arthroplasty using trabecular titanium implants. Int Orthop 2015;39:389-95. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty 1994;9:33-44. [Crossref] [PubMed]
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712-6. [Crossref] [PubMed]
- Patel I, Nham F, Zalikha AK, et al. Epidemiology of total hip arthroplasty: demographics, comorbidities and outcomes. Arthroplasty 2023;5:2. [Crossref] [PubMed]
- Malahias MA, Sarantis M, Gkiatas I, et al. The modern Burch-Schneider antiprotrusio cage for the treatment of acetabular defects: is it still an option? A systematic review. Hip Int 2023;33:705-15. [Crossref] [PubMed]
- Fryhofer GW, Ramesh S, Sheth NP. Acetabular reconstruction in revision total hip arthroplasty. J Clin Orthop Trauma 2020;11:22-8. [Crossref] [PubMed]
- Yang C, Zhu K, Dai H, et al. Mid- to Long-term Follow-up of Severe Acetabular Bone Defect after Revision Total Hip Arthroplasty Using Impaction Bone Grafting and Metal Mesh. Orthop Surg 2023;15:750-7. [Crossref] [PubMed]
- Gillinov SM, Joo PY, Zhu JR, et al. Incidence, Timing, and Predictors of Hip Dislocation After Primary Total Hip Arthroplasty for Osteoarthritis. J Am Acad Orthop Surg 2022;30:1047-53. [Crossref] [PubMed]
- Alberton GM, High WA, Morrey BF. Dislocation after revision total hip arthroplasty: an analysis of risk factors and treatment options. J Bone Joint Surg Am 2002;84:1788-92. [Crossref] [PubMed]
- Grosso MJ, Kozaily E, Cacciola G, et al. Characterizing Femoral and Acetabular Bone Loss in Two-Stage Revision Total Hip Arthroplasty for Infection. J Arthroplasty 2021;36:311-6. [Crossref] [PubMed]
- Mancino F, Cacciola G, Di Matteo V, et al. Surgical implications of the hip-spine relationship in total hip arthroplasty. Orthop Rev (Pavia) 2020;12:8656. [Crossref] [PubMed]
- Abdel MP, von Roth P, Jennings MT, et al. What Safe Zone? The Vast Majority of Dislocated THAs Are Within the Lewinnek Safe Zone for Acetabular Component Position. Clin Orthop Relat Res 2016;474:386-91. [Crossref] [PubMed]
- Lewinnek GE, Lewis JL, Tarr R, et al. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am 1978;60:217-20. [Crossref] [PubMed]
- Berlinberg EJ, Roof MA, Shichman I, et al. Prior Instability is Strongly Associated With Dislocation After Isolated Head and Liner Exchange. J Arthroplasty 2022;37:2412-9. [Crossref] [PubMed]
- Hanslmeier MG, Maier MW, Feisst M, et al. Femoral Head and Liner Exchange in Patients with Atraumatic Dislocation. Results of a Retrospective Study with 6 Years Follow-Up. Medicina (Kaunas) 2021;57:1188. [Crossref] [PubMed]
- Bruschetta D, Anastasi G, Andronaco V, et al. Human calf muscles changes after strength training as revealed by diffusion tensor imaging. J Sports Med Phys Fitness 2019;59:853-60. [Crossref] [PubMed]
- Bosco F, Cacciola G, Giustra F, et al. Characterizing recurrent infections after one-stage revision for periprosthetic joint infection of the knee: a systematic review of the literature. Eur J Orthop Surg Traumatol 2023;33:2703-15. [Crossref] [PubMed]
Cite this article as: Cacciola G, Giustra F, Bosco F, De Meo F, Bruschetta A, De Martino I, Risitano S, Sabatini L, Massè A, Cavaliere P. Trabecular titanium cups in hip revision surgery: a systematic review of the literature. Ann Joint 2023;8:36.



