
Preoperative planning in reverse shoulder arthroplasty: plain radiographs vs. computed tomography scan vs. navigation vs. augmented reality
Introduction
Reverse shoulder arthroplasty (RSA) is a highly successful treatment option for improving pain and function in several conditions of the shoulder including irreparable massive rotator cuff tears, rotator cuff arthropathy, complex proximal humerus fractures, prosthetic revisions, end-stage glenohumeral arthritis, and severe bone erosion (1). As a result, the use of RSA in the United States has markedly increased over time since its approval in 2003 (2-4). Despite the increasing popularity of RSA, several complications may occur postoperatively including scapular notching, hematoma formation, prosthetic instability, component loosening, infection, and acromial or scapular spine fracture (5-7).
Some of these complications may be related to technical errors during component implantation and thus they may be prevented with proper preoperative planning and accurate position of the prosthetic components. Several studies have demonstrated that improper baseplate and glenosphere position in RSA may result in impingement, reduced range of motion and increased scapular notching (8-11). The relationship between component positioning and stability of the RSA has been also demonstrated. In a biomechanical study, Favre et al. examined the resistance to anterior dislocation of RSA implants with varying degrees of version of the humerus and glenoid components (12). The findings of this study indicated that the version of the humeral component version is the crucial factor for intrinsic stability (i.e., stability of the implants regardless of soft tissues or other patient-related factors). However, it should be noted that the version of the glenoid component might also play a role, particularly when retroversion exceeds 10 degrees.
Adequate initial glenoid baseplate fixation achieved by optimal baseplate seating on native bone and screws of adequate length and trajectory is crucial to avoid loosening and early failure (13-17). Other factors related with implant positioning and RSA configuration that may influence outcomes and complications, such as scapular notching, neurological injuries, or acromial fractures, are the amount of lateralization and distalization achieved postoperatively (18,19). However, there is still a lack of established golden rules to plan these parameters preoperatively (20).
Despite the influence of component position on outcomes and complications after RSA, glenoid component implantation is challenging for surgeons and glenoid position errors have been found to be common with standard instrumentation even in cases performed by experienced surgeons (21,22). Several factors contribute to the difficulty for glenoid positioning in RSA including deformity, bone defects, difficulty with exposure and the scarcity of reliable bony landmarks to assess the glenoid vault and the scapular plane intraoperatively.
Given the increasing knowledge on the biomechanics of RSA and the impact of implant configuration and positioning on outcomes and complications, preoperative planning and surgical aids that improve implant position accuracy have been developed over the last decade. The aim of this review is to review and describe the current evidence on preoperative planning in RSA from plain radiographs, three-dimensional (3D) imaging techniques and the use of computer planning software, to the most recent technological advancements such as intraoperative navigation and augmented reality (AR).
Plain radiographs
The traditional radiographic evaluation of patients with glenohumeral arthritis includes the typical views of a shoulder series [anteroposterior, Grashey (“true” anteroposterior), scapular Y, and axillary views]. These radiographs are useful to help evaluate joint space narrowing, osteophytes, posterior glenoid wear, and version as well as to stage the comprise of the joint by the underlying condition and make the differential diagnosis between conditions (i.e., primary glenohumeral osteoarthritis versus cuff tear arthropathy). Traditionally, surgeons relied on plain radiographs and intraoperative findings to assess glenoid morphology and implant the components of shoulder arthroplasty. However, this method can be unreliable specially in cases with more severe deformity (23). Nowadays, preoperative planning of RSA based solely on plain radiographs is an option for selected cases with no or minimal glenoid deformity, and surgeons must acknowledge the limitations of this approach.
Radiographs are prone to variability in measurements since the image of the scapula on the X-ray film depends greatly on the orientation of the X-ray beam relative to the plane of the scapula during examination. As a result, radiographic measurements of glenoid version, glenoid inclination and humeral subluxation are inaccurate. In a study of 25 patients, Nyffeler et al. demonstrated that glenoid retroversion was overestimated on plain radiographs in 86% of cases, with a maximum difference of 21° (mean, 6.4°) compared with the values measured on computed tomography (CT) scans (24). These authors demonstrated in radiographs of anatomical specimens that small variations in the alignment of the X-ray beam or in the position of the patient can result in substantial changes in the measured glenoid version (24). In another study, Mulligan et al. also demonstrated that radiographs provided significantly less precise measurements of glenoid version and highlighted the detrimental effect of high body mass index on the observers’ ability to judge classifications, especially on radiographs (25). Daggett et al. demonstrated that the beta angle, used to measure glenoid inclination, was less accurate and reliable on the AP radiograph than in the reformatted two-dimensional (2D) CT scans (26).
The Walch classification is the most accepted method to assess glenoid morphology and wear patterns in patients with glenohumeral osteoarthritis (27). However, classification of morphologic features of the glenoid in the original Walch description is based on axial cuts of 2D CT scans. Kopka et al. evaluated in a study of 50 patients whether the Walch classification could be accurately applied to X-ray images compared with magnetic resonance imaging (MRI) as the gold standard (28). These authors concluded that radiographs are significantly inferior to cross-sectional imaging when applying the Walch classification and thus recommend that advanced imaging be part of the standard preoperative assessment for shoulder arthroplasty.
Analog or manual templating has been used in joint replacements for many years. In shoulder arthroplasty, magnified templates available from manufacturers can be superimposed onto calibrated plain radiographs for preoperative planning. The available evidence for manual templating is limited to anatomic total shoulder arthroplasty and shows that templating is only accurate for stem size (29). Since there is no way of manual templating glenoid baseplate positioning, which is the most critical aspect in RSA preoperative planning, there is limited utility of manual templating for RSA.
Several radiographic measurements have been proposed in the setting of RSA including the center of rotation, critical shoulder angle, lateral humeral offset, acromial index, acromiohumeral interval and deltoid lever arm (30,31). While the change of these parameters between preoperative and postoperative may have a potential association with clinical outcomes or complications, there is a lack of validation of these measurements and their role in the preoperative planning of RSA has yet to be established.
2D CT imaging
As previously stated, 2D CT provides a better detail of bony pathology and allows a more accurate estimation of important parameters such as glenoid version, glenoid inclination, and humeral subluxation than plain radiographs (10,26,28). Despite these advantages, it has yet to be determined if advanced imaging is necessary for preoperative planning in every case of shoulder arthroplasty. Liuzza et al. (32) performed a study to determine if addition of CT to axillary radiographs alters preoperative decision making for shoulder arthroplasty. They found that axillary radiographs are often inadequate for preoperative planning in shoulder arthritis with advanced glenoid wear patterns (Walch A2, B2, C types), and in these cases with advanced glenoid wear, addition of CT can change the preoperative plan with respect to arthroplasty type and/or strategy for addressing glenoid wear.
Considering that the scapula is typically oriented in 20° to 30° of anteversion with respect to the coronal plane, an important caveat when using 2D CT images to estimate glenoid version and inclination is that the plane of axial reconstruction should be aligned with the scapula instead of the patient’s torso. Several studies have shown that the accuracy of 2D CT is dependent on the angle of axial reconstruction in relation to the position of the scapula. Bryce et al. showed that any malalignment of at least 1°of the scapula in the coronal or sagittal plane will create inaccuracies in measuring glenoid version (33). Hoenecke Jr et al. showed that clinical CT scans were axially aligned with the patient’s torso but were almost never perpendicular to the scapular body (34). In addition, they found that the point of greatest wear was missed on 2D scans in 52% of cases and absolute error in version measured on the 2D CT slice passing through the tip of the coracoid was 5.1°, while in 20% of cases, the error was >10°. Bokor et al. showed that the measured value for glenoid version according to the Friedman method varied as much as 10° on the same specimen with minor rotations of the scapula (35). For cases with Walch B2-type glenoids, if 2D CT images are not reoriented into the plane of the scapula, version and inclination will be significantly overestimated (36).
In conclusion, 2D CT imaging may be useful to better assess bony pathology specially in cases with more severe deformity, classify the glenoid morphology according to the original Walch classification (27), and determine glenoid version and inclination according to described methods (26,37). However, it is critical to take into consideration the limitations of this method and the need for proper CT reconstruction orientation perpendicular to the scapular body.
3D CT imagining and preoperative planning software programs
Given the limitations of 2D CT imaging to accurately reflect the 3D anatomy of the glenoid, 3D imaging and analysis of the scapula as a free body gained popularity for preoperative planning of RSA. Initially, 3D models for templating were created by manually reformatting 2D CT images. From these manually segmented 3D models, accurate methods to measure glenoid version were described (38,39). Kwon et al. demonstrated that glenoid measurements made from a 3D CT scan were within 1.0°±0.7° (mean ± standard deviation) of those from the cadaver scapula that was scanned (38). This evidence supported the use of 3D CT scan imaging as the gold-standard method for determining a patient’s glenoid anatomy. However, there is criticism and concerns with manual segmentation of 2D CT images to create 3D models which include variations in the location of the anatomical landmarks used to define planes due to glenoid deformity or osteophytes, and the logistical inconveniences in terms of technology availability, costs, time, and expertise if surgeons decide to implement this method in their practices.
Several studies have compared 3D CT versus 2D CT imaging in terms of glenoid measurements and understanding of glenoid morphology. In a systematic review of the literature including six studies comparing 2D CT to 3D CT planning in shoulder arthroplasty, Olaiya et al. found that the variability in glenoid measurements between 3D CT and 2D CT planning ranged from no significant difference to a 5° in version and 1.7° difference in inclination (40). Additionally, they found posterior bone loss was underestimated in 52% of the 2D measured patients relative to 3D CT groups. Although this evidence supports that 3D planning technology results in different radiographic measurements when compared to standard 2D planning, these differences were quite small and at times, unlikely to be clinically significant with limited reliability. Furthermore, it cannot be ascertained that such differences represent improved evaluation given the lack of a gold-standard (40).
With the advent of 3D CT imaging, the evolution of preoperative planning software programs has exploded in recent years with multiple implant systems offering their own preoperative planning programs. These programs reconstruct a 3D model from a standard thin slice (1 mm) 2D CT using different methods. Then, the software uses automated and semiautomated algorithms to help identify anatomical landmarks and estimate glenoid version, glenoid inclination, and in some of the available programs, posterior head subluxation, humeral version, humeral inclination, and humeral head size. In addition, these programs enable virtual implantation of the glenoid and humeral components into the 3D models. Depending on the software, other parameters can be assessed and simulated including central pin guide placement, baseplate seating on native bone, peripheral screws length and trajectory, the need for augmented implant, impingement, and range of motion. Main features and measurement methods of the different preoperative planning software programs currently available are presented in Table 1.
Table 1
| Designer (preoperative software) | Main features | Estimation model | Glenoid center | Glenoid reference points | Scapular plane | Transverse axis |
|---|---|---|---|---|---|---|
| Medacta® (My Shoulder®) | Single-use PSI: humeral cutting guide, glenoid guide | Glenoid-face model | Most medial point | 4 points: anterior, posterior, inferior, superior | 3 points: glenoid center, inferior scapular angle, and scapular trigonum | Glenoid center-trigonum scapulae |
| Glenoid and humeral planning | ||||||
| Simulates postoperative range of motion of the joint | ||||||
| Simulates postoperative humeral displacement (distalization, anteriorization and lateralization) | ||||||
| Estimates reaming depth and % bone-implant contact | ||||||
| Navigated augmented reality technology | ||||||
| 3D measurements: humeral retroversion, posterior subluxation, glenoid inclination, glenoid version | ||||||
| DJO Surgical®-Enovis® (Match Point System®-Materialise®) | Single-use PSI: glenoid guide | Glenoid-face model | Geometric center (automated) | All points of glenoid fossa | 3 points: glenoid center, inferior scapular angle, and scapular trigonum | Supraspinatus fossa line |
| Provide a drill path | ||||||
| Only glenoid planning | ||||||
| 3D measurements: glenoid inclination and version | ||||||
| Tornier-Wright® (BLUEPRINT®) | Single-use PSI: glenoid guide | Best-fit sphere model | Geometric center (automated) | All points of glenoid fossa | Best fit plane of scapula using a regression model that includes all points of scapular body (automated) | Y-axis (cross section between scapula and scapular spine) |
| Glenoid and humeral planning | ||||||
| Simulates postoperative range of motion of the joint | ||||||
| Estimates reaming depth and % bone-implant contact | ||||||
| 3D measurements: posterior subluxation, glenoid inclination, glenoid version | ||||||
| Zimmer-Biomet® (Signature ONE Planner®) | Single-use PSI: glenoid guide | Glenoid-face model | Crossing of inferior-superior and anterior-posterior axes | 3 points: anterior, superior, posterior | 3 points: glenoid center, inferior scapular angle, and scapular trigonum | Glenoid center-trigonum scapulae |
| Only glenoid planning | ||||||
| 3D measurements: glenoid version and inclination | ||||||
| Central screw length | ||||||
| Estimates percentage of implant contact | ||||||
| Estimates medial/lateral translation of the baseplate | ||||||
| 3D measurements: glenoid version and inclination | ||||||
| Exactech® (Shoulder GPS®) | Navigation technology | Glenoid-face model | Average of the most superior inferior, anterior, and posterior points on the glenoid | 4 points: anterior, posterior, inferior, superior | 3 points: glenoid center, inferior scapular angle, and scapular trigonum | Glenoid center-trigonum scapulae |
| Glenoid and humeral planning | ||||||
| Select from a range of humeral stem components | ||||||
| Alter humeral trays and liners in RSA | ||||||
| Simulates shoulder range of motion and impingement | ||||||
| Estimates lateralization and distalization | ||||||
| Estimates reaming depth and bone-implant contact (no contact, contact, contact >2 mm) | ||||||
| Option to manually adjust the points selected for determining version and inclination | ||||||
| 3D measurements: glenoid version and inclination | ||||||
| Arthrex® (Virtual Implant Planning VIP®) | Reusable PSI: glenoid side | Vault model | NR | 3 points: superior, anteroinferior, posteroinferior | 3 points: glenoid center, inferior scapular angle, and scapular trigonum | Glenoid center-trigonum scapulae |
| Planning only the glenoid side | ||||||
| Estimates percentage of implant contact | ||||||
| Estimates medial/lateral translation of the baseplate | ||||||
| Estimates screw trajectory and length | ||||||
| Estimates the center of rotation | ||||||
| 3D measurements: glenoid version and inclination | ||||||
| Lima Corporate® (Smart Space®) | Single-use PSI: humeral cutting guide, glenoid guide | Glenoid-face model | NR | 2 points: superior and inferior point | 3 points: glenoid center, inferior scapular angle, and scapular trigonum | Glenoid center-trigonum scapulae |
| Planning of both glenoid and humerus | ||||||
| Estimates reaming depth and % bone-implant contact | ||||||
| Navigation technology (smart Space® cubit sensors) | ||||||
| 3D measurements: glenoid version, glenoid inclination, humerus version and neck angle | ||||||
| Stryker® (TrueSight®-Materialise®) | Single-use PSI: glenoid guide | Glenoid-face model | Geometric center (automated) | All points of glenoid fossa | 3 points: glenoid center, inferior scapular angle, and scapular trigonum | Supraspinatus fossa line |
| Planning only the glenoid side | ||||||
| Estimates percentage of implant contact | ||||||
| Estimates medial/lateral translation of the baseplate | ||||||
| Estimates screw trajectory and length | ||||||
| 3D measurements: glenoid version and inclination | ||||||
| Depuy Synthes® (TruMatch®-Materialise®) | Single-use PSI: glenoid guide | Glenoid-face model | Geometric center (automated) | All points of glenoid fossa | 3 points: glenoid center, inferior scapular angle, and scapular trigonum | Supraspinatus fossa line |
| Planning of both glenoid and humerus | ||||||
| Estimates maximum erosion depth | ||||||
| Estimates vault loss | ||||||
| Simulates impingement and range of motion | ||||||
| Estimates screw trajectory and length | ||||||
| 3D measurements: glenoid version and inclination. Humeral head diameter and posterior head subluxation |
3D, three-dimensional; PSI, patient-specific instrumentation; RSA, reverse shoulder arthroplasty; NR, not reported.
It is essential to understand the measurement techniques each planning software utilizes to estimate anatomic parameters as there is not a standardized coordinate system defined and different reference points are used to define the glenoid and scapular planes. The vast majority of the currently available preoperative software programs use landmark-based models (Figure 1). In these models, the glenoid-face plane, scapular plane, and transverse axis used to determine anatomic parameters are based on manually selected anatomical landmarks (Table 1). The BLUEPRINT® software is the only program that uses the best-fit sphere model (Figure 1). In this model, a totally automated algorithm mathematically creates a best-fit sphere incorporating data from all points of the glenoid surface to estimate the glenoid plane and glenoid centerline. Similarly, the scapular plane is mathematically estimated using a regression model that incorporates all points of scapular body. Shah et al. compared glenoid measurements from these two models (landmark-based model and best-fit sphere model) against a control computed tomography-derived 3D printed scapula and found that a high percentage of cases showed discrepancies in glenoid inclination and version values from both models (41).
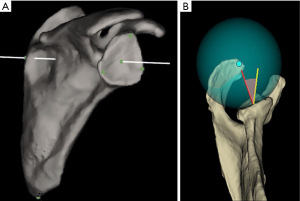
Multiple studies demonstrate significant differences in measurement techniques concerning preoperative glenoid anatomy between commercially available planning programs (42-46). This variability results in discrepancies in measures of version and inclination and limited agreement of these parameters between programs (Table 2). Overall, all preoperative planning programs overestimate version, inclination, and subluxation as compared to manual measurements, with BLUEPRINT® demonstrating the lowest agreement with surgeon measurements (42). This may be due to differences in the geometrical algorithm and mathematical calculations this software employs as explained before. In addition to differences in the reference coordinate system, glenoid deformity, presence of osteophytes, labral calcifications, and CT artifacts are all key contributors to morphological measurement inconsistencies (45). Since most of the studies do not have a true-anatomy gold-standard to which compare software measurements, it is currently unknown which method better approximates true anatomy and provides potentially a more valuable guide for glenoid implantation.
Table 2
| Study | Year | Cases | Parameters evaluated | Comparisons | Results |
|---|---|---|---|---|---|
| Denard et al. (46) | 2018 | 63 patients | Glenoid version and inclination | BLUEPRINT® and VIP® | Version: difference <5° in 70% of cases and ≥5° in 30% of cases |
| Inclination: difference <5° in 54% of cases and ≥5° in 46% of cases | |||||
| Erickson et al. (42) | 2021 | 81 patients | Glenoid version, inclination, subluxation | BLUEPRINT®, VIP®, Materialise®, ExactechGPS® and 5 surgeons manual measurements | Significant differences were found between surgeon and commercial software measurements in version, inclination, and subluxation. Software measurements tended to be more superiorly inclined, more retroverted, and more posteriorly subluxed than surgeon measurements |
| Waltz et al. (44) | 2022 | 30 patients | Glenoid version and inclination | BLUEPRINT®, VIP®, and 2 surgeons manual measurements | Version within 5° =47% of cases |
| Glenoid region at which version and inclination are measured | Inclination within 5° =63% of cases | ||||
| Location of version measurement different among 2 software programs (VIP® superior and BLUEPRINT® inferior) | |||||
| Forneau et al. (43) | 2022 | 13 cadaveric shoulders | Glenoid version and inclination | BLUEPRINT®, Signature ONE®, Materialise®, MyShoulder® and 2 investigator methods | Inclination: ranged from 0.38° (SignatureOne® Zimmer-Biomet) to 10.31° (BLUEPRINT® Tornier-Wright) |
| Version: the differences in the measurement of version were more subtle, yet sometimes still statistically significant. The mean version ranged from 3.54° (MyShoulder® Medacta) to 0.54° (SignatureOne® Zimmer-Biomet) | |||||
| Webb et al. (45) | 2022 | 76 patients | Glenoid version and inclination | VIP®, BLUEPRINT®, True-Sight®, ExactechGPS® and 2 radiologist manual measurements | Measurements of glenoid version and inclination differed between at least 2 programs by 5°–10° in 75% and 92% of glenoids respectively, and by >10° in 18% and 45% respectively. When measuring version, VIP® had the highest concordance with manual measurement; BLUEPRINT® had the lowest. For inclination BLUEPRINT® had the highest concordance; ExactechGPS® had the lowest |
Similarly, while the clinical relevance of these differences is unknown, they may heavily influence the decision regarding the type of arthroplasty and reconstruction plan especially if specific quantitative guidelines are applied. Surgeons should be aware of these differences and the decisions taken during preoperative planning based on the anatomic measurements provided by the preoperative planning programs. Further research is needed to better understand how this variability should be accounted for during preoperative planning for RSA.
The influence of 3D preoperative planning in the understanding of glenoid pathology and implant selection has been evaluated in several studies. Werner et al. in a study that included 50 patients undergoing shoulder arthroplasty found that decision on the choice of implant was adjusted in 7 patients (14%) after the 3D planning (47). Rosenthal et al. compared two cohorts undergoing shoulder arthroplasty with and without 3D preoperative planning and found an almost three-fold increase in the use of augmented components when using 3D preoperative planning (15% 2D CT vs. 54% 3D CT) (48). Similarly, Nashikkar et al. demonstrated that patients undergoing computer-assisted shoulder arthroplasty had more than twice as many augmented glenoid components as the conventional group (49). However, none of these studies presented costs or clinical outcomes comparisons and thus it cannot be ascertained whether the use of augmented components was a cost-effective intervention or not.
Min et al. evaluated whether 3D preoperative planning, when compared with the use of plain radiographs or select static CT images, influenced the understanding of glenoid pathology and surgical planning in a case-based survey presented to 59 surgeons (50). They found that for all surgeons, the use of 3D preoperative planning increased agreement with the experts in glenoid classification and surgical planning; preoperative planning had the greatest impact on the surgical decision-making of low volume surgeons. Tashjian et al. showed that 3D computer assisted planning without patient specific instrumentation in the setting of RSA with severe glenoid erosion requiring bone grafting can accurately guide baseplate placement (51). All cases in which failure to correct retroversion or inclination within 10° of planning occurred in patients with severe erosion (B3 or E3 glenoids), and thus these authors recommend the use of patient specific guides in these cases.
Other potential benefits of 3D preoperative planning are decreasing costs and increasing efficiency in the operating room due to the improved accuracy in predicting intraoperative implant selection in RSA. Raiss et al. found a complete concordance between the preoperative plan and final implant selection in 90% of RSA cases (52). These authors suggest this high concordance may assist with surgical preparedness, implant stocks, and possibly future implant production. Sheth et al. in a retrospective study comparing patients who underwent shoulder arthroplasty with and without 3D preoperative found that while preoperative planning did not reduce time in the operating room, it was correlated to a significant reduction in the number and cost of sterilized trays (53).
In conclusion, 3D preoperative planning has improved the understanding of glenoid pathology and virtual templating may be helpful for surgeons especially in cases with more severe glenoid deformity. However, there are inconsistencies in the estimation of anatomic glenoid measurements between planning systems and therefore, surgeons should be careful when making decisions based solely on computer planning measurements and when comparing publications that use different planning systems to determine preoperative glenoid deformity measurements. To have a less biased evaluation, it is recommended that in addition to preoperative software evaluation surgeons analyze advanced imaging studies independently and assess glenoid anatomy free of any software influence.
Patient-specific instrumentation (PSI) and navigation
PSI was developed aiming to improve the accuracy of the intraoperative implementation of virtual preoperative surgical planning. Schoch et al. showed that despite preoperative planning, surgeons of various training levels were unable to reproducibly replicate the planned component position consistently (54). Most implant companies offer a single use customized glenoid guide based on the preoperative plan (Table 1). The Medacta My Shoulder® and the Lima Corporate SmartSpace® systems also offer a single use guide for the humerus osteotomy. The Arthrex VIP® is the only system with a reusable guide for PSI and the Zimmer SignatureONE® system is the only one that provides a glenoid guide with an end point for reaming depth. Figure 2 shows different PSI guides from multiple implant companies.
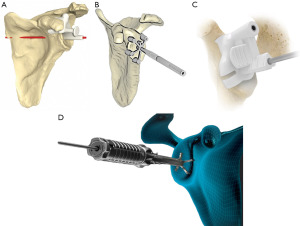
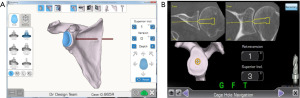
Computer navigation has been developed to assist intraoperatively in the position of the glenoid component as planned preoperatively without the need of PSI guides. The only commercially available navigation system for RSA is the ExactechGPS®, which is used with the Exactech Equinoxe® shoulder system (Figure 3). The GPS system uses a fixed point in the patient’s anatomy combined with input from the surgeon of several anatomic landmarks to create a 3D mapping of the joint. All instruments are then referenced to this 3D mapping and based on preoperative plan, intraoperative navigation provides real-time feedback for guide pin placement, central peg drilling and reaming, and peripheral screws placement. All this information is displayed on a separate monitor. One concern with intraoperative navigation is the learning curve for surgeons in the use of this technology and the increased surgical times. A study on intraoperative computer navigation of the glenoid component showed that with prior surgeon training, navigation does not substantially increase operating times compared with standard surgical techniques, and it was estimated that approximately 8 operative cases are required to achieve proficiency in the use of this technology (55).
Several studies have determined the improved accuracy of glenoid component implantation with the use of PSI or navigation. In a systematic review of the literature that included nine articles evaluating the concordance between 3D preoperative planning and actual implant in RSA, Lilley et al. found that preoperative planning combined with PSI or navigation led to baseplate version and inclination deviation of less than 5° in all studies (56). Studies published after this systematic review show similar results (57-60) (Table 3). In addition to glenoid position, patients who had preoperative planning combined with PSI or navigation had accurate screw placement using 2 or 4 screws on the glenoid component (58,60,61). Accurate screw placement is critical for optimal baseplate fixation and to prevent damage to soft tissues and neurovascular structures in the shoulder. Kwak et al. demonstrated that when using PSI there was a significant decrease in the proportion of screws involving the spinoglenoid notch (58). A systematic review of the literature that included six studies evaluating the influence of intraoperative navigation on the length and number of screws in RSA showed that intraoperative navigation improves the baseplate screw placement, allowing for a greater screw purchase length and fewer screws to achieve primary fixation (62).
Table 3
| Study | Number of cases | Technology evaluated | Mean deviation from planned version | Mean deviation from planned inclination |
|---|---|---|---|---|
| Nashikkar et al. (49)* | 33 | Navigation | 81.8% of cases within 5° of plan | 75.8% of cases within 5° of plan |
| Schoch et al. (54) | 37 | Navigation | 6.4°±5.6° | 6.6°±4.9° |
| Dallalana et al. (57) | 10 | PSI | 1.8°±1.9° | 1.3°±1.0° |
| Kwak et al. (58) | 39 | PSI | 1.8°±1.2° | 1.7°±1.2° |
| Marcoin et al. (59) | 35 | PSI | 2.3°±1.0 | 2.4°±2.0° |
| Verborgt et al. (60) | 32 | PSI | 4.4°±3.1° | 5°±4.2° |
*, means values were not presented but the percentage of cases within 5° of plan. PSI, patient-specific instrumentation.
Most of the technology and evidence on PSI and navigation for RSA has focused on positioning of the glenoid component and few studies have evaluated PSI or navigation for humerus osteotomy. Suter et al. showed that a preoperative planning of the standard osteotomy technique along the anterosuperior anatomic neck using a 3D CT model is accurate within a threshold of 10° when using a free-hand technique in 92% of cases for inclination (63). However, retroversion and resected head thickness differed from the preoperative plan, thereby limiting the unrestricted use of humeral head osteotomy planning from 3D CT models in shoulder arthroplasty. Similarly, the accuracy of implant prediction for the humeral component is not as good as that described for the glenoid component. Wittmann et al. showed that the concordance of planned to implanted stem size and tray offset for RSA was only 44.2% and 65%, respectively (64). Despite the low concordance between planned and implanted stem size, the choice of stem size was found to be in range of one adjacent size in 87.6% of cases. Rojas et al. compared the accuracy of humeral cutting PSI guides and standard cutting guides in cadaveric specimens (65). They found that while PSI and standard guides had similar accuracy for inclination, PSI had less deviation between planned and postosteotomy humeral retrotorsion and height, relative to standard guides. Cavanagh et al. evaluated in a preclinical study the use of navigation for humerus osteotomy and compared its accuracy for executing a planned humerus osteotomy with that of PSI (66). No significant differences were found between PSI guides and navigation for recreation of the preoperatively planned humeral head cut height and version. Neck-shaft angle (i.e., angle between the angle of the long axis of the humerus and the normal of the osteotomy plane), however, had significantly less deviation from the preoperative plan when conducted with navigation. Further studies are needed to assess the clinical accuracy of PSI and the application of navigation for humerus osteotomy as well as to define the thresholds at which changes in inclination, retrotorsion and height could influence clinical outcomes.
AR
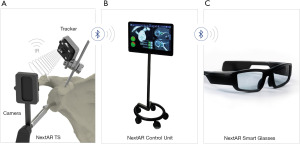
Another technology that has been developed to aid the surgeon in executing their preoperative plan during surgery is navigated AR. As it is the case for traditional navigation, the main advantages of navigated AR over PSI are that it obviates the need for physical guides and allows real-time visualization of the plan intraoperatively providing real-time feedback to the surgeon. Navigated AR allows the real world to be augmented with virtual real-time information regarding the position and orientation of instruments and glenoid component (67). Unlike traditional navigation, in which imaging is displayed on a separate monitor, in navigated AR essential information is presented directly overlaid onto the surgical field through a head-mounted display allowing the surgeon to stay focused on the patient. The only commercially available navigated AR system for RSA is the NextAR™ shoulder AR surgical platform which is used with the Medacta® Shoulder system (Figure 4). Preclinical feasibility and cadaveric studies of this technology have confirmed its accuracy and reliability for glenoid component placement (68,69). No clinical studies have been published so far on the accuracy or clinical outcomes of navigated AR.
Conclusions
In conclusion, PSI, navigation, and navigated AR are all useful technological advances that aid the surgeon to improve accuracy and reliability of glenoid component positioning. However, further research is needed to determine the added value of these technological advances in terms of improving clinical outcomes for the patients. The scarce evidence comparing short-term clinical outcomes of RSA with and without the use of PSI or navigation show no or marginal benefits of these technologies (53,70,71). Any benefit should be balanced against the increased costs and the 200 to 1,000-fold increase in radiation exposure associated with the CT scans in comparison with radiographs (72). In this regard, a recent study by Lorenzana et al. demonstrated that simulated low-dose CT images were sufficient for reliable measurement of glenoid version, glenoid inclination, and humeral head subluxation by preoperative planning software, as well as by physician-observers, suggesting a potential for substantial reduction in radiation dose for preoperative shoulder CT scans (73). In addition, more research is also needed to establish optimal planning parameters to improve the reproducibility among surgeons in glenoid baseplate positioning during 3D planning as current evidence demonstrate that there is little agreement on the lateralization, version and inclination criteria for positioning the glenoid baseplate between surgeons (20,74).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Prashant Meshram) for the series “Controversies in Shoulder Surgery and Algorithmic Approach to Decision Making” published in Annals of Joint. The article has undergone external peer review.
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-20/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-20/coif). The series “Controversies in Shoulder Surgery and Algorithmic Approach to Decision Making” was commissioned by the editorial office without any funding or sponsorship. GF receives payment or honoraria for lectures, presentations, speakers bureaus from Stryker and Arthrex. GF also receives support for attending meetings and/or travel from Stryker and Arthrex. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Familiari F, Rojas J, Nedim Doral M, et al. Reverse total shoulder arthroplasty. EFORT Open Rev 2018;3:58-69. [Crossref] [PubMed]
- Best MJ, Aziz KT, Wilckens JH, et al. Increasing incidence of primary reverse and anatomic total shoulder arthroplasty in the United States. J Shoulder Elbow Surg 2021;30:1159-66. [Crossref] [PubMed]
- Farley KX, Wilson JM, Kumar A, et al. Prevalence of Shoulder Arthroplasty in the United States and the Increasing Burden of Revision Shoulder Arthroplasty. JB JS Open Access 2021;6:e20.00156.
- Weber SC, Rojas J, Meshram P, et al. Reverse shoulder arthroplasty: trends in the incidence, indications and complications as reported by ABOS part II oral examination candidates from 2005 through 2017. Semin Arthroplasty 2021;31:299-309. [Crossref]
- Barco R, Savvidou OD, Sperling JW, et al. Complications in reverse shoulder arthroplasty. EFORT Open Rev 2016;1:72-80. [Crossref] [PubMed]
- Galvin JW, Kim R, Ment A, et al. Outcomes and complications of primary reverse shoulder arthroplasty with minimum of 2 years' follow-up: a systematic review and meta-analysis. J Shoulder Elbow Surg 2022;31:e534-44. [Crossref] [PubMed]
- Zumstein MA, Pinedo M, Old J, et al. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146-57. [Crossref] [PubMed]
- Gutiérrez S, Comiskey CA 4th, Luo ZP, et al. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am 2008;90:2606-15. [Crossref] [PubMed]
- Kolmodin J, Davidson IU, Jun BJ, et al. Scapular Notching After Reverse Total Shoulder Arthroplasty: Prediction Using Patient-Specific Osseous Anatomy, Implant Location, and Shoulder Motion. J Bone Joint Surg Am 2018;100:1095-103. [Crossref] [PubMed]
- Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005;14:524-8. [Crossref] [PubMed]
- Werner BS, Chaoui J, Walch G. Glenosphere design affects range of movement and risk of friction-type scapular impingement in reverse shoulder arthroplasty. Bone Joint J 2018;100-B:1182-6. [Crossref] [PubMed]
- Favre P, Sussmann PS, Gerber C. The effect of component positioning on intrinsic stability of the reverse shoulder arthroplasty. J Shoulder Elbow Surg 2010;19:550-6. [Crossref] [PubMed]
- Chebli C, Huber P, Watling J, et al. Factors affecting fixation of the glenoid component of a reverse total shoulder prothesis. J Shoulder Elbow Surg 2008;17:323-7. [Crossref] [PubMed]
- Parsons BO, Gruson KI, Accousti KJ, et al. Optimal rotation and screw positioning for initial glenosphere baseplate fixation in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:886-91. [Crossref] [PubMed]
- Torkan LF, Bryant JT, Bicknell RT, et al. Central fixation element type and length affect glenoid baseplate micromotion in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2022;31:1385-92. [Crossref] [PubMed]
- Abdic S, Lockhart J, Alnusif N, et al. Glenoid baseplate screw fixation in reverse shoulder arthroplasty: does locking screw position and orientation matter? J Shoulder Elbow Surg 2021;30:1207-13. [Crossref] [PubMed]
- DiStefano JG, Park AY, Nguyen TQ, et al. Optimal screw placement for base plate fixation in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2011;20:467-76. [Crossref] [PubMed]
- Kim HJ, Kwon TY, Jeon YS, et al. Neurologic deficit after reverse total shoulder arthroplasty: correlation with distalization. J Shoulder Elbow Surg 2020;29:1096-103. [Crossref] [PubMed]
- Boutsiadis A, Lenoir H, Denard PJ, et al. The lateralization and distalization shoulder angles are important determinants of clinical outcomes in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2018;27:1226-34. [Crossref] [PubMed]
- Berhouet J, Jacquot A, Walch G, et al. Preoperative planning of baseplate position in reverse shoulder arthroplasty: Still no consensus on lateralization, version and inclination. Orthop Traumatol Surg Res 2022;108:103115. [Crossref] [PubMed]
- Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg Am 2012;94:2167-75. [Crossref] [PubMed]
- Gregory TM, Sankey A, Augereau B, et al. Accuracy of glenoid component placement in total shoulder arthroplasty and its effect on clinical and radiological outcome in a retrospective, longitudinal, monocentric open study. PLoS One 2013;8:e75791. [Crossref] [PubMed]
- Iannotti JP, Greeson C, Downing D, et al. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:48-55. [Crossref] [PubMed]
- Nyffeler RW, Jost B, Pfirrmann CW, et al. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg 2003;12:493-6. [Crossref] [PubMed]
- Mulligan RP, Feldman JJ, Bonnaig NS, et al. Comparison of axillary lateral radiography with computed tomography in the preoperative characterization of glenohumeral wear patterns and the effects of body mass index on quality of imaging. Curr Orthop Pract 2019;30:471-6. [Crossref]
- Daggett M, Werner B, Gauci MO, et al. Comparison of glenoid inclination angle using different clinical imaging modalities. J Shoulder Elbow Surg 2016;25:180-5. [Crossref] [PubMed]
- Vo KV, Hackett DJ, Gee AO, et al. Classifications in Brief: Walch Classification of Primary Glenohumeral Osteoarthritis. Clin Orthop Relat Res 2017;475:2335-40. [Crossref] [PubMed]
- Kopka M, Fourman M, Soni A, et al. Can glenoid wear be accurately assessed using x-ray imaging? Evaluating agreement of x-ray and magnetic resonance imaging (MRI) Walch classification. J Shoulder Elbow Surg 2017;26:1527-32. [Crossref] [PubMed]
- Buzzell JE, Lutton DM, Shyr Y, et al. Reliability and accuracy of templating the proximal humeral component for shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:728-33. [Crossref] [PubMed]
- Kim DH, Choi HU, Choi BC, et al. Postoperative acromiohumeral interval affects shoulder range of motions following reverse total shoulder arthroplasty. Sci Rep 2022;12:21011. [Crossref] [PubMed]
- Berthold DP, Morikawa D, Muench LN, et al. Negligible Correlation between Radiographic Measurements and Clinical Outcomes in Patients Following Primary Reverse Total Shoulder Arthroplasty. J Clin Med 2021;10:809. [Crossref] [PubMed]
- Liuzza LG, Abdelshahed MM, Oh C, et al. Comparison of radiographs and computed tomography (CT) imaging for preoperative evaluation and planning for shoulder arthroplasty. Seminars in Arthroplasty JSES 2021;31:395-401.
- Bryce CD, Davison AC, Lewis GS, et al. Two-dimensional glenoid version measurements vary with coronal and sagittal scapular rotation. J Bone Joint Surg Am 2010;92:692-9. [Crossref] [PubMed]
- Hoenecke HR Jr, Hermida JC, Flores-Hernandez C, et al. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg 2010;19:166-71. [Crossref] [PubMed]
- Bokor DJ, O'Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg 1999;8:595-8. [Crossref] [PubMed]
- Chalmers PN, Salazar D, Chamberlain A, et al. Radiographic characterization of the B2 glenoid: the effect of computed tomographic axis orientation. J Shoulder Elbow Surg 2017;26:258-64. [Crossref] [PubMed]
- Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am 1992;74:1032-7. [Crossref] [PubMed]
- Kwon YW, Powell KA, Yum JK, et al. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg 2005;14:85-90. [Crossref] [PubMed]
- Lewis GS, Armstrong AD. Glenoid spherical orientation and version. J Shoulder Elbow Surg 2011;20:3-11. [Crossref] [PubMed]
- Olaiya OR, Nadeem I, Horner NS, et al. Templating in shoulder arthroplasty - A comparison of 2D CT to 3D CT planning software: A systematic review. Shoulder Elbow 2020;12:303-14. [Crossref] [PubMed]
- Shah SS, Sahota S, Denard PJ, et al. Variability in total shoulder arthroplasty planning software compared to a control CT-derived 3D printed scapula. Shoulder Elbow 2021;13:268-75. [Crossref] [PubMed]
- Erickson BJ, Chalmers PN, Denard P, et al. Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg 2021;30:413-20. [Crossref] [PubMed]
- Fourneau T, van Haute E, De Wilde L, et al. 3D preoperative planning for shoulder arthroplasty: an evaluation of different planning software systems. Semin Arthroplasty 2022;32:474-81. [Crossref]
- Waltz RA, Peebles AM, Ernat JJ, et al. Commercial 3-dimensional imaging programs are not created equal: version and inclination measurement positions vary among preoperative planning software. JSES Int 2022;6:413-20. [Crossref] [PubMed]
- Webb AR, Bodendorfer BM, Laucis NC, et al. Significant variability exists in preoperative planning software measures of glenoid morphology for shoulder arthroplasty. Semin Arthroplasty 2022;32:82-92. [Crossref]
- Denard PJ, Provencher MT, Lädermann A, et al. Version and inclination obtained with 3-dimensional planning in total shoulder arthroplasty: do different programs produce the same results? JSES Open Access 2018;2:200-4.
- Werner BS, Hudek R, Burkhart KJ, et al. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1477-83. [Crossref] [PubMed]
- Rosenthal Y, Rettig SA, Virk MS, et al. Impact of preoperative 3-dimensional planning and intraoperative navigation of shoulder arthroplasty on implant selection and operative time: a single surgeon's experience. J Shoulder Elbow Surg 2020;29:2564-70. [Crossref] [PubMed]
- Nashikkar PS, Scholes CJ, Haber MD. Computer navigation re-creates planned glenoid placement and reduces correction variability in total shoulder arthroplasty: an in vivo case-control study. J Shoulder Elbow Surg 2019;28:e398-409. [Crossref] [PubMed]
- Min KS, Fox HM, Bedi A, et al. Patient-specific planning in shoulder arthroplasty. Bone Joint J 2020;102-B:365-70. [Crossref] [PubMed]
- Tashjian RZ, Beck L, Stertz I, et al. Preoperative three-dimensional computer planning for reverse total shoulder arthroplasty and bone grafting for severe glenoid deformity. Shoulder Elbow 2021;13:492-501. [Crossref] [PubMed]
- Raiss P, Walch G, Wittmann T, et al. Is preoperative planning effective for intraoperative glenoid implant size and type selection during anatomic and reverse shoulder arthroplasty? J Shoulder Elbow Surg 2020;29:2123-7. [Crossref] [PubMed]
- Sheth B, Lavin AC, Martinez C, et al. The use of preoperative planning to decrease costs and increase efficiency in the OR. JSES Int 2022;6:454-8. [Crossref] [PubMed]
- Schoch BS, Haupt E, Leonor T, et al. Computer navigation leads to more accurate glenoid targeting during total shoulder arthroplasty compared with 3-dimensional preoperative planning alone. J Shoulder Elbow Surg 2020;29:2257-63. [Crossref] [PubMed]
- Wang AW, Hayes A, Gibbons R, et al. Computer navigation of the glenoid component in reverse total shoulder arthroplasty: a clinical trial to evaluate the learning curve. J Shoulder Elbow Surg 2020;29:617-23. [Crossref] [PubMed]
- Lilley BM, Lachance A, Peebles AM, et al. What is the deviation in 3D preoperative planning software? A systematic review of concordance between plan and actual implant in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2022;31:1073-82. [Crossref] [PubMed]
- Dallalana RJ, McMahon RA, East B, et al. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg 2016;10:59-66. [Crossref] [PubMed]
- Kwak JM, Jeon IH, Kim H, et al. Patient-specific instrumentation improves the reproducibility of preoperative planning for the positioning of baseplate components with reverse total shoulder arthroplasty: a comparative clinical study in 39 patients. J Shoulder Elbow Surg 2022;31:1488-98. [Crossref] [PubMed]
- Marcoin A, Nerot C, Lestra T, et al. The precision of patient-specific instrumentation guides for the positioning of the glenoid component in total reverse shoulder arthroplasty: an in vivo scanographic study. Int Orthop 2020;44:1761-6. [Crossref] [PubMed]
- Verborgt O, Hachem AI, Eid K, et al. Accuracy of patient-specific guided implantation of the glenoid component in reversed shoulder arthroplasty. Orthop Traumatol Surg Res 2018;104:767-72. [Crossref] [PubMed]
- Moreschini F, Colasanti GB, Cataldi C, et al. Pre-Operative CT-Based Planning Integrated With Intra-Operative Navigation in Reverse Shoulder Arthroplasty: Data Acquisition and Analysis Protocol, and Preliminary Results of Navigated Versus Conventional Surgery. Dose Response 2020;18:1559325820970832. [Crossref] [PubMed]
- Velasquez Garcia A, Abdo G. Does computer-assisted navigation improve baseplate screw configuration in reverse shoulder arthroplasty? A systematic review and meta-analysis of comparative studies. J Orthop 2023;36:29-35. [Crossref] [PubMed]
- Suter T, Gerber Popp A, Kolz CW, et al. Accuracy of free-hand humeral head resection planned on 3D-CT models in shoulder arthroplasty: an in vitro analysis. Arch Orthop Trauma Surg 2022;142:3141-7. [Crossref] [PubMed]
- Wittmann T, Befrui N, Rieger T, et al. Stem size prediction in shoulder arthroplasty with preoperative 3D planning. Arch Orthop Trauma Surg 2023;143:3735-41. [Crossref] [PubMed]
- Rojas JT, Jost B, Hertel R, et al. Patient-specific instrumentation reduces deviations between planned and postosteotomy humeral retrotorsion and height in shoulder arthroplasty. J Shoulder Elbow Surg 2022;31:1929-37. [Crossref] [PubMed]
- Cavanagh J, Lockhart J, Langohr GDG, et al. A comparison of patient-specific instrumentation to navigation for conducting humeral head osteotomies during shoulder arthroplasty. JSES Int 2021;5:875-80. [Crossref] [PubMed]
- Rojas JT, Lädermann A, Ho SWL, et al. Glenoid Component Placement Assisted by Augmented Reality Through a Head-Mounted Display During Reverse Shoulder Arthroplasty. Arthrosc Tech 2022;11:e863-74. [Crossref] [PubMed]
- Kriechling P, Loucas R, Loucas M, et al. Augmented reality through head-mounted display for navigation of baseplate component placement in reverse total shoulder arthroplasty: a cadaveric study. Arch Orthop Trauma Surg 2023;143:169-75. [Crossref] [PubMed]
- Kriechling P, Roner S, Liebmann F, et al. Augmented reality for base plate component placement in reverse total shoulder arthroplasty: a feasibility study. Arch Orthop Trauma Surg 2021;141:1447-53. [Crossref] [PubMed]
- Elsheikh AA, Galhoum MS, Mokhtar MA, et al. Patient-specific Instrumentation Versus Standard Surgical Instruments in Primary Reverse Total Shoulder Arthroplasty: A Retrospective Comparative Clinical Study. J Shoulder Elb Arthroplast 2022;6:24715492221075449. [Crossref] [PubMed]
- Holzgrefe RE, Hao KA, Panther EJ, et al. Early clinical outcomes following navigation-assisted baseplate fixation in reverse total shoulder arthroplasty: a matched cohort study. J Shoulder Elbow Surg 2023;32:302-9. [Crossref] [PubMed]
- Schiffman CJ, Prabhakar P, Hsu JE, et al. Assessing the Value to the Patient of New Technologies in Anatomic Total Shoulder Arthroplasty. J Bone Joint Surg Am 2021;103:761-70. [Crossref] [PubMed]
- Lorenzana DJ, Solomon J, French RJ, et al. Comparison of Simulated Low-Dose and Conventional-Dose CT for Preoperative Planning in Shoulder Arthroplasty. J Bone Joint Surg Am 2022;104:1004-14. [Crossref] [PubMed]
- Parsons M, Greene A, Polakovic S, et al. Assessment of surgeon variability in preoperative planning of reverse total shoulder arthroplasty: a quantitative comparison of 49 cases planned by 9 surgeons. J Shoulder Elbow Surg 2020;29:2080-8. [Crossref] [PubMed]
Cite this article as: Rojas J, Lievano , Jiménez AM, González-Rico HA, Salas M, Fierro G, González JC. Preoperative planning in reverse shoulder arthroplasty: plain radiographs vs. computed tomography scan vs. navigation vs. augmented reality. Ann Joint 2023;8:37.


