Risk factors for recurrence of periprosthetic joint infection following operative management: a cohort study with average 5-year follow-up
Introduction
Total joint replacement (TJR) is the gold standard treatment for end-stage hip and knee osteoarthritis (OA) (1). Overall, TJR surgeries are among the most successful and cost-effective healthcare interventions worldwide (2). Nonetheless, as with any major surgery, there is a small but significant risk of complications. Medical complications include venous thromboembolism, myocardial infection, and delirium. Hardware-related complications, on the other hand, include infection, loosening, and polyethylene wear (3-5). The rates of many hardware-related complications, such as dislocation, periprosthetic fracture, wear and loosening have decreased over time due to improved technology and surgical techniques (6-8). Infection rates, however, have not changed substantially, and may even be increasing (9,10).
Periprosthetic joint infection (PJI) is a devastating complication of TJR surgery—given that there is no direct blood supply to the implant, oral or intravenous antibiotics are rarely sufficient to treat PJI (11). The vast majority of cases require one or more surgeries, and in rare cases can eventually lead to fusion, amputation, disarticulation, and even death (12). In addition to the obvious personal burden of PJI on patients, there is also a massive societal impact to be considered. Revision surgeries cost nearly 80% more than primary surgeries, and in 2017–2018 the total inpatient cost of revision TJR surgery in Canada was $163 million, not including physician payments or rehabilitation costs (13). Infection is the leading cause of revision surgery, and the rates of revision surgery in Canada increased by about 10% from 2012 to 2018 (13).
Currently, the treatment of PJI involves operative management with a prolonged course of postoperative antibiotics (14). Operative management ranges from debridement, antibiotics, and implant retention (DAIR) with or without revision of modular implants, to one- and two-stage revision arthroplasty, and in some cases, excision arthroplasty, fusion, or disarticulation (14). Even with appropriate surgical management, PJIs remain challenging to eradicate and recurrence rates following revision surgery may be close to 30% (15). Previous studies have identified risk factors for PJI recurrence including patient characteristics, causative organism, chronicity of infection and operative intervention performed (16-18). However, the majority of the literature on the topic consists of small sample sizes with short term follow-up which may underestimate the true recurrence rate (16,19,20). Additionally, many cohorts do not include patients who have undergone the full range of surgical options utilized in the management of PJI (15-17). There is a need for studies examining large patient cohorts who have undergone the full range of operative interventions to understand risks of recurrence in this population.
Thus, the purpose of this study is to determine the predictors of PJI recurrence in patients undergoing operative management for PJI at a high-volume academic arthroplasty centre, and to determine differences in recurrence free survival between DAIR and staged revision procedures. We present the following article in accordance with the STROBE reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-4/rc) (21).
Methods
The database cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Hamilton Integrated Research Board (HiREB #10633). Given that this was a retrospective chart review using only de-identified data, consent was not obtained from the individual patients.
Study design
This cohort study was designed as a retrospective analysis of prospectively collected data, though the data was not collected specifically for this study.
Setting
This study was based on an arthroplasty database at an academic hospital in Ontario, Canada. The dates included in the study were 2011 to 2018, inclusive. The hospital is an arthroplasty hub for a public, academic health network serving about 2.3 million people in the surrounding regions. All follow-up data available on or before February 20, 2021 were included for analysis.
Participants
All participants undergoing revision surgery for PJI following hip or knee arthroplasty between 2011 and 2018 were included. These dates were selected as our experience with revision surgery for PJI in this patient population prior to 2010 has been previously published (22). Participants treated with non-operative measures only were excluded, as were those for whom information about the index surgery was unavailable. Follow-up data was collected prospectively and added to the database in the course of routine clinical follow-up as well as from any visits to the emergency department.
Variables
Data was collected on patient sex, age, type of index surgery, lab values [pre-operative C-reactive protein (CRP), synovial fluid cell count], presence of a chronic sinus tract, use of antibiotic cement and local intrawound antibiotics, and type of infective organism (methicillin-resistant vs. not). The primary outcome was a confirmed recurrence of deep PJI, categorized as a dichotomous variable.
Data sources/measurement
The prospective database used in this study has collected data on all arthroplasty surgeries performed at the hospital since 1998 and includes over 23,000 patients. Sex was characterized as a dichotomous variable, while age and lab values were measured as continuous variables. The remaining variables were categorical. Patients were censored at time of a repeat revision surgery.
Bias
In order to ensure accurate diagnoses, an infectious disease specialist reviewed all patients identified as having had potential PJIs and any patients who were unlikely to have had a true PJI based on the full clinical picture were excluded from analysis. As well, the data analysis methodology was determined prior to pulling the relevant data from the database.
Study size
Given that data from the database’s PJI cohort had been previously published, all data available since 2011 was included. A post-hoc power calculation was performed to ensure that the sample size was sufficient to detect the demonstrated differences.
Statistical analysis
Multivariable logistic regression analysis was utilized to determine the relationship between the predictor variables (age, sex, index surgery, CRP, synovial cell count, presence of a chronic sinus tract, use of antibiotic cement, use of intrawound antibiotics, and type of infective organism) and the outcome variable (need for revision surgery). The relationship between each predictor variable and the outcome was analyzed in a univariate regression analysis, and any variables with a P value of <0.25 were included in a single final logistic regression model (23). Furthermore, a survival analysis with log rank testing was used to compare recurrence-free survival between patients who underwent DAIR with liner exchange and those who underwent the first stage of a two-stage revision. Results are presented as mean [standard deviation (SD)] where appropriate, and P<0.05 was considered to be statistically significant.
Results
Participants
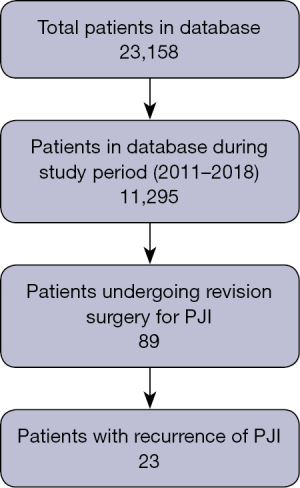
A total of 11,295 patients were included in the database in the period between 2011 and 2018, among whom 89 patients (91 joints) were found to have undergone revision surgery due to PJI (Figure 1).
Descriptive data
Forty-one knees (39 patients) and 50 hips (50 patients) were included, of whom 37 were males (41.6%) and 52 were females (58.4%). Mean age at time of revision surgery was 68.8 (11.2) years, and the median time between index and revision surgery was 34 days (range 2 days to 8.7 years). Most patients (65/91, 71.4%) were treated with DAIR, while 24.2% (22/91) were treated with a staged revision with cement spacer, 3 patients (3.3%) were treated with excision arthroplasty, and one patient (1.1%) was treated with concomitant irrigation and debridement and open reduction internal fixation for pelvic discontinuity. Of the patients treated with DAIR, the majority (54/65, 83.1%) also underwent revision of modular components (i.e., femoral heads, polyethylene liners, etc.), while the remaining (11/65, 16.9%) had all implants retained. Some of these patterns, such as the lack of any single stage revisions and retaining some modular components were related to surgeon preference, particularly in the earlier part of the cohort, and do not necessarily reflect the current standard of care at our institution. Twenty-one patients (23.1%) passed away over the study period. None of the deaths were directly related to a PJI or perioperative complications, or were being worked up for a suspected or confirmed PJI in the period immediately prior to their death. All patients had a documented date of death and note available. Mean follow-up was 5.6 (2.5) years (range: 2 months to 10.1 years). Interestingly, rates of MRSA infection were similar between patients treated with DAIR (7/58, 12.1%), and those treated with staged revision (2/20, 10%). See Table 1 for a summary of demographic data.
Table 1
| Variables | Value |
|---|---|
| Age (years), mean (SD) | 68.8 (11.2) |
| Pre-operative CRP, mean (SD) | 122.6 (115.1) |
| Presence of chronic sinus tract, N (%) | |
| Yes | 15 (16.5%) |
| No | 69 (75.8%) |
| Unclear | 7 (7.7%) |
| MRSA infection, N (%) | |
| Yes | 9 (9.9%) |
| No | 72 (79.1%) |
| Unknown | 10 (11.0%) |
| Sex, N (%) | |
| Male | 37 (41.6%) |
| Femail | 52 (58.4%) |
| Joint, N (%) | |
| Knee | 41 (45.1%) |
| Hip | 50 (54.9%) |
SD, standard deviation; N, number; CRP, C-reactive protein; MRSA, methicillin-resistant Staphylococcus aureus.
Outcome data
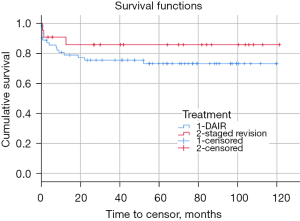
Mean recurrence-free survival post-revision surgery was 7.9 years [95% confidence interval (CI): 7 to 8.7 years]. Survival was not significantly different between patients who underwent DAIR versus those who underwent a staged revision with cumulative survival rates of 73.3% and 85.9%, respectively (P=0.27) (Figure 2). The median time between the revision surgery for first PJI and repeat revision surgery was 89 days (range: 7 days to 5.9 years). Cumulative survival rate of patients undergoing DAIR without modular component exchange was 60.0%, though these cases were not analyzed separately due to this not being a pre-planned analysis, small sample size, and concern about multiple comparisons.
Main results
Based on univariate analyses, age (χ2=5.52, P=0.019) and presence of a sinus tract (χ2=5.33, P=0.021) were found to be statistically significant for risk of PJI recurrence, and were the only two variables below the pre-determined threshold of P<0.25 (Table 2). A multivariable logistic regression model including both of these variables was significant for predicting recurrence of PJI (χ2=10.2, P=0.006). The model was 92.3% specific, but only 21.1% sensitive for predicting recurrence of PJI. The odds ratio (OR) for recurrence of PJI in patients with a chronic draining sinus was 4.89, while older patients were at lower risk of PJI recurrence (OR 0.56 for each additional decade of older age).
Table 2
| Independent variable | χ2 | P value |
|---|---|---|
| Sex | 0.639 | 0.424 |
| Joint | 0.591 | 0.442 |
| Method of treatment | 0.970 | 0.325 |
| Pre-operative C-reactive protein | 0.062 | 0.804 |
| Synovial cell count | 0.517 | 0.472 |
| Presence of chronic sinus tract | 4.165 | 0.041 |
| Age at revision surgery | 4.211 | 0.040 |
| Use of antibiotic cement | 0.667 | 0.414 |
| Use of intrawound antibiotics (other than cement) | 0.062 | 0.804 |
| Methicillin-resistant Staphylococcus aureus (MRSA) infection | 0.001 | 0.973 |
PJI, periprosthetic joint infection.
Other analyses
A post-hoc power analysis revealed that based on the use of two independent variables, the comparative OR presented, and the corresponding event rates, a sample size of 76 would have been needed to achieve 90% power at the P=0.05 level. Thus, our sample size was sufficiently powered to detect the differences presented above.
Discussion
Key results
The key findings of this study are that younger age and the presence of a chronic sinus tract significantly predict recurrence of PJI. Both variables were independent predictors of risk of recurrence, and a model including both variables is highly specific for doing so. In addition, there is relatively high recurrence-free survivorship, which was higher among those treated with staged revision compared to DAIR, though not significantly so. The survival rates in this study are similar to previously published cohorts of similar size (24,25).
Older age was protective against recurrence of PJI in the model, and was independently predictive of a lower risk of recurrence. Multiple potential explanations may exist for this finding. Older age has traditionally been postulated as a risk factor for PJI and recurrent PJI, due to its association with co-morbidities and frailty (26). However, upon closer inspection, multiple large studies have demonstrated that younger age is actually associated with significantly higher risk of PJI and recurrence of PJI (27,28). In fact, in a retrospective cohort study of 23,966 patients, Inoue et al. demonstrated that when controlling for confounding factors through propensity score matching analysis, age was actually not significantly associated with risk of PJI (29).
The appropriateness of the DAIR strategy has been debated, and its effectiveness remains unclear in the broader literature. A systematic review and meta-analysis of 93 studies which treated a total of 4,897 patients with DAIR found an extremely variable range of recurrence rates, from 0% to 89%; the pooled estimated recurrence rate was 39.6% (30). Our study demonstrated a considerably lower recurrence rate of 26.5% for patients treated with DAIR, which is in line with some recent literature on the DAIR strategy (31,32). Part of this seeming discrepancy may arise from the appropriateness of patient selection for DAIR—a previous study from our database found that when patients were treated with DAIR according to the 2013 Musculoskeletal Infection Society (MSIS) guidelines, there was a 100% cure rate, compared to only 44.4% cure rate in patients who did not fit the criteria for DAIR treatment as per the guidelines (33). Though new MSIS guidelines were published in 2018 (34), careful patient selection undoubtedly remains crucial to successful treatment of all PJIs, and perhaps even more so when DAIR is the treatment strategy of choice. One of the frequently cited indications for DAIR is a low virulence infective organism (35). Though rates of MRSA infection were similar in patients treated with DAIR and staged revision in our study, speciated cultures were frequently not available pre-operatively.
An important area of study which continues to require further investigation is the duration of post-operative antibiotics, as well as the role of chronic suppressive antibiotics (24). In this study, as with many institutions, infectious disease physicians made decisions on a case-by-case basis, with some loosely defined standard of care patterns. However, given recent level 1 evidence showing that there may be a benefit to the use of intravenous antibiotics for 12 weeks compared to 6 weeks for the treatment of PJIs (36), this is an area that demands further study and standardization. Furthermore, the role of chronic suppressive antibiotics remains unclear, and no clear guidelines exist for selection of patients who may benefit from this form of treatment either as definitive treatment or in conjunction with surgical management. A recent systematic review found there is still only low-quality evidence to determine the role of suppressive antibiotics in the management of PJI.
Interestingly, the median time to initial PJI was about one month following index surgery, whereas the median time from revision TJR to repeat surgery among the recurrent PJI cohort was about three months. This may be in part due to the course of post-operative intravenous antibiotics provided in PJI cases, which at our institution is typically six weeks. Nonetheless, this confirms that roughly half of both first-time and recurrent PJIs occur within the first three months after surgery. A recent 15-year population-based study in a Canadian cohort found that in patients undergoing primary THA surgery, the median time to PJI is about 18 months, which again confirms that about half of all PJIs occur early in the post-operative period (9). The discrepancy in specific timelines is likely due to the longer term follow-up in that study.
Limitations
The limitations of this study include the fact that it is derived from a single centre, and thus the study may lack generalizability. In addition, though the data was collected prospectively, our analysis was retrospective, which inevitably introduces potential sources of bias. In addition, a sizeable proportion of patients passed away during the course of the study; while this is not unexpected given the patient population, it does introduce some risk of bias. As well, our model was highly specific, but had limited sensitivity for ruling out recurrent PJI. Finally, though the database is regularly maintained, validated, and updated, it is nonetheless a single-centre database that relies on accurate reporting, and is limited to healthcare interactions with the network of affiliated hospitals. Thus, there may be unknown missing data.
Strengths
This study is drawn from a large, well-maintained database at a high-volume academic centre, and was adequately powered to detect differences based on the sample size. It analyzes outcomes following the full range of PJI treatment strategies and infection data was independently verified by an infectious disease specialist, which helps to minimize diagnostic bias associated with retrospective analysis. As well, the recurrence rate in this cohort was consistent with our experience from 2005–2010, demonstrating internal validity of the database. Finally, this study provides medium-term follow-up on a patient population that can be difficult to study over longer term periods.
Interpretation
Younger patients and those with a chronic sinus tract prior to revision surgery are at significantly higher risk of recurrent PJI, and thus may require differential counselling and closer post-operative monitoring. This study demonstrates that PJI can be successfully managed in a majority of cases with DAIR or staged revision.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-4/rc
Data Sharing Statement: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-4/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-4/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The database cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Hamilton Integrated Research Board (HiREB #10633). Given that this was a retrospective chart review using only de-identified data, consent was not obtained from the individual patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mandl LA. Determining who should be referred for total hip and knee replacements. Nat Rev Rheumatol 2013;9:351-7. [Crossref] [PubMed]
- Bumpass DB, Nunley RM. Assessing the value of a total joint replacement. Curr Rev Musculoskelet Med 2012;5:274-82. [Crossref] [PubMed]
- Abbas K, Murtaza G, Umer M, et al. Complications of total hip replacement. J Coll Physicians Surg Pak 2012;22:575-8.
- Healy WL, Iorio R, Clair AJ, et al. Complications of Total Hip Arthroplasty: Standardized List, Definitions, and Stratification Developed by The Hip Society. Clin Orthop Relat Res 2016;474:357-64. [Crossref] [PubMed]
- Healy WL, Della Valle CJ, Iorio R, et al. Complications of total knee arthroplasty: standardized list and definitions of the Knee Society. Clin Orthop Relat Res 2013;471:215-20. [Crossref] [PubMed]
- Dawson-Amoah K, Raszewski J, Duplantier N, et al. Dislocation of the Hip: A Review of Types, Causes, and Treatment. Ochsner J 2018;18:242-52. [Crossref] [PubMed]
- Kurtz SM, Muratoglu OK, Evans M, et al. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials 1999;20:1659-88. [Crossref] [PubMed]
- Abu-Amer Y, Darwech I, Clohisy JC. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res Ther 2007;9:S6. [Crossref] [PubMed]
- McMaster Arthroplasty Collaborative (MAC). Risk Factors for Periprosthetic Joint Infection Following Primary Total Hip Arthroplasty: A 15-Year, Population-Based Cohort Study. J Bone Joint Surg Am 2020;102:503-9. [Crossref] [PubMed]
- Perfetti DC, Boylan MR, Naziri Q, et al. Have Periprosthetic Hip Infection Rates Plateaued? J Arthroplasty 2017;32:2244-7. [Crossref] [PubMed]
- Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev 2019;4:482-94. [Crossref] [PubMed]
- Tande AJ, Gomez-Urena EO, Berbari EF, et al. Management of Prosthetic Joint Infection. Infect Dis Clin North Am 2017;31:237-52. [Crossref] [PubMed]
- Canadian Institute for Health Information. Hip and Knee Replacements in Canada, 2017–2018: Canadian Joint Replacement Registry Annual Report. Canadian Institute for Health Information. Ottawa, ON; 2019. Available online: https://secure.cihi.ca/free_products/cjrr-annual-report-2019-en-web.pdf (Accessed Feb 21, 2021).
- Kapadia BH, Berg RA, Daley JA, et al. Periprosthetic joint infection. Lancet 2016;387:386-94. [Crossref] [PubMed]
- Kandel CE, Jenkinson R, Daneman N, et al. Predictors of Treatment Failure for Hip and Knee Prosthetic Joint Infections in the Setting of 1- and 2-Stage Exchange Arthroplasty: A Multicenter Retrospective Cohort. Open Forum Infect Dis 2019;6:ofz452. [Crossref] [PubMed]
- Citak M, Friedenstab J, Abdelaziz H, et al. Risk Factors for Failure After 1-Stage Exchange Total Knee Arthroplasty in the Management of Periprosthetic Joint Infection. J Bone Joint Surg Am 2019;101:1061-9. [Crossref] [PubMed]
- Mortazavi SM, Vegari D, Ho A, et al. Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. Clin Orthop Relat Res 2011;469:3049-54. [Crossref] [PubMed]
- Zmistowski B, Fedorka CJ, Sheehan E, et al. Prosthetic joint infection caused by gram-negative organisms. J Arthroplasty 2011;26:104-8. [Crossref] [PubMed]
- Kubista B, Hartzler RU, Wood CM, et al. Reinfection after two-stage revision for periprosthetic infection of total knee arthroplasty. Int Orthop 2012;36:65-71. [Crossref] [PubMed]
- Hsieh PH, Lee MS, Hsu KY, et al. Gram-negative prosthetic joint infections: risk factors and outcome of treatment. Clin Infect Dis 2009;49:1036-43. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- Winemaker M, Mertz D, Petruccelli D, et al. Prosthetic joint infections: Is guideline-consistent surgical treatment beneficial? Curr Orthop Pract 2018;29:560-4.
- Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008;3:17. [Crossref] [PubMed]
- Petis SM, Abdel MP, Perry KI, et al. Long-Term Results of a 2-Stage Exchange Protocol for Periprosthetic Joint Infection Following Total Hip Arthroplasty in 164 Hips. J Bone Joint Surg Am 2019;101:74-84. [Crossref] [PubMed]
- Xu C, Tan TL, Chen JY. Positive Culture During Reimplantation Increases the Risk of Reinfection in Two-Stage Exchange Arthroplasty Despite Administrating Prolonged Antibiotics: A Retrospective Cohort Study and Meta-Analysis. J Arthroplasty 2019;34:1025-31. [Crossref] [PubMed]
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med 2015;3:233. [Crossref] [PubMed]
- Kurtz SM, Lau E, Schmier J, et al. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty 2008;23:984-91. [Crossref] [PubMed]
- Lee QJ, Mak WP, Wong YC. Risk factors for periprosthetic joint infection in total knee arthroplasty. J Orthop Surg (Hong Kong) 2015;23:282-6. [Crossref] [PubMed]
- Inoue D, Xu C, Yazdi H, et al. Age alone is not a risk factor for periprosthetic joint infection. J Hosp Infect 2019;103:64-8. [Crossref] [PubMed]
- Kunutsor SK, Beswick AD, Whitehouse MR, et al. Debridement, antibiotics and implant retention for periprosthetic joint infections: A systematic review and meta-analysis of treatment outcomes. J Infect 2018;77:479-88. [Crossref] [PubMed]
- Barros LH, Barbosa TA, Esteves J, et al. Early Debridement, antibiotics and implant retention (DAIR) in patients with suspected acute infection after hip or knee arthroplasty - safe, effective and without negative functional impact. J Bone Jt Infect 2019;4:300-5. [Crossref] [PubMed]
- Grammatopoulos G, Kendrick B, McNally M, et al. Outcome Following Debridement, Antibiotics, and Implant Retention in Hip Periprosthetic Joint Infection—An 18-Year Experience. J Arthroplasty 2017;32:2248-55. [Crossref] [PubMed]
- Parvizi J, Gehrke TInternational Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty 2014;29:1331. [Crossref] [PubMed]
- Parvizi J, Tan TL, Goswami K, et al. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty 2018;33:1309-1314.e2. [Crossref] [PubMed]
- Azzam KA, Seeley M, Ghanem E, et al. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty 2010;25:1022-7. [Crossref] [PubMed]
- Bernard L, Arvieux C, Brunschweiler B, et al. Antibiotic Therapy for 6 or 12 Weeks for Prosthetic Joint Infection. N Engl J Med 2021;384:1991-2001. [Crossref] [PubMed]
Cite this article as: Ekhtiari S, Gazendam A, Saidahmed A, Petruccelli D, Winemaker MJ, de Beer JD, Shah V, Wood TJ. Risk factors for recurrence of periprosthetic joint infection following operative management: a cohort study with average 5-year follow-up. Ann Joint 2023;8:2.