
Venous thromboembolism in musculoskeletal oncology surgery
Introduction
Venous thromboembolism (VTE), including both deep venous thrombosis (DVT) and pulmonary embolism (PE), is a common complication observed in patients with metastatic bone disease (MBD). Despite the well-known association between cancer and VTE, the mechanism promoting this pathology is not entirely well understood. In cancer patients, all three aspects of Virchow’s triad, hemodynamic stasis, endothelial injury, and hypercoagulability, are likely involved in the promotion of thromboembolic events (1). In many of these patients, tumors may compress veins with resultant stasis and cause a highly hypoxic environment that damages endothelium. Additionally, abnormalities in the coagulation and fibrinolytic pathways as well as platelet activation have been implicated in VTE, and excitation or suppression of these specific pathways may be tumor dependent (2). Additional factors such as radiation therapy to extremities causing vessel effects may compound these risks. It is estimated that nearly all cancer patients will experience from some form of VTE, whether or not clinically relevant, during the course of their disease. Annually, the incidence of VTE in cancer patients is 0.5%, much higher than the 0.1% in the general population (3). There are many risk factors for VTE including increased age, female sex, black and Hispanic race, comorbid disease, immobility, and previous history of VTE (2). Cancer associated thrombosis may be impacted by various risk factors including a cancer site, advanced stage, histological subtype, and the time since diagnosis (4). Additionally, hospitalization time, central venous catheter, and treatments such as chemotherapy and surgery are well-established risk factors for VTE (5).
Numerous studies have analyzed the occurrence and prevention of VTE in patients with cardiovascular disease or suffering trauma (6), but very few have specifically examined the safety or efficacy of preventing VTE in cancer patients with metastatic skeletal disease. After the lungs and liver, bone is the third most common site of cancer metastases (7). The risk of VTE in patients undergoing intramedullary nailing for metastatic disease involving long bones is known to be high (8). In a retrospective review of the risk of VTE in patients undergoing intramedullary nailing for prophylactic fixation related to skeletal metastases, 203 of 336 patients (60.4%) received low molecular weight heparin (LMWH), the most frequently used anticoagulant, followed by 44.6% enoxaparin, 15.8% dalteparin, 16.7% warfarin, 5.4% subcutaneous heparin, 5.1% aspirin, 0.9% fondaparinux, 0.6% unverifiable and 11.0% no anticoagulant. In total, 24 VTE events occurred [13 PEs (3.9%) and 11 DVTs (3.3%)]. Although the data reflected that LMWH was the most commonly used anticoagulant in patients that developed VTE, further data analysis determined that there was no correlation between the type of anticoagulant used and the occurrence of VTE (8) in this patient population.
This study highlights both the need for and the inconsistencies in choice of anticoagulation in this population. This review will examine the various types of prophylactic treatment, timing of administration, risk stratification for determining the appropriate course of anticoagulation (AC) and discuss current views on chemical prophylaxes relativity to wound complications and excessive bleeding.
Mechanical, chemical and combination prophylaxis
Cancer and orthopedic surgery are known risk factors for VTE (9), and therefore the need for prophylactic treatment aimed at preventing the occurrence of VTE in these patient populations is well understood (10). However, the ideal choice of prophylactic treatment for patients undergoing surgery for skeletal metastases is unknown.
Mechanical prophylaxis
Mechanical prophylaxis, either alone or in combination with chemical prophylaxis, in patients undergoing orthopedic surgery is widely accepted due to minimal risk of serious complications with use (11). Woolson et al. studied the use of intermittent pneumatic sequential compression devices (SCD) to prevent proximal DVT during and after hip replacement. Patients were randomized to one of three cohorts: SCD alone, SCD plus aspirin, or SCD plus low dose heparin, in an effort to examine the true efficacy of mechanical prophylaxis alone or in combination with chemical prophylaxis. The results of the study supported the argument that mechanical prophylaxis alone was effective, safe and convenient with respect to preventing proximal DVT during and after hip replacement (11). However, although partial or total hip replacement is a common procedure in patients with MBD, the results of this study may not be applicable to the MBD population. Lin et al. conducted a prospective study assessing the risk of VTE in patients undergoing major orthopedic surgery of the lower limb for which knee-high pneumatic compression stockings were used prophylactically in combination with ultrasound screening. The rate of symptomatic PE was low, but the risk of DVT was significant (10). In fact, the risk of proximal DVT was substantial even when combined with chemical prophylaxis (10). One study addresses the use of mechanical prophylaxis alone in patients who underwent resection of musculoskeletal tumors of the lower limb: DVT was detected in 21 (22%) patients of the 94 patients who underwent resection of a lower limb musculoskeletal tumor. In addition, one patient experienced a fatal PE. The conclusion was drawn that the use of monotherapy mechanical prophylaxis was an insufficient means of preventing VTE in this population (12).
Chemical prophylaxis
Surgery for malignant disease increases the risk of VTE nearly two-fold compared to patients undergoing non-cancer related orthopaedic surgery (13). Additionally, patients with cancer are more likely to experience recurrence of VTE than those without (13). Thus, since about 2004 all major orthopaedic patients were recommended to receive postoperative chemoprophylaxis (14). However, which type of chemical prophylaxis remains widely debated both for efficacy and safety.
Vitamin K antagonists (VKAs), such as warfarin, were once considered the gold standard for VTE prevention. The risk of VTE was felt to outweigh the potential for excessive bleeding complications, so patients received VKAs both post-operatively as well as for conventional long-term management of VTE. However, the extensive list of challenges associated with the administration of VKAs have subsequently led to the increasing popularity of other options. VKAs demonstrate a known established risk of an increased anticoagulant effect when combined with other concomitant therapies (13). There is a long delay between the initiation of therapy and the observed anticoagulant effect, which in some cases may require or prolong an inpatient hospital stay (13). Whether or not VTE occurs, the administration of VKAs long-term post-operatively requires continuous lab monitoring (13). The patient’s location may play a role in non-compliance by making it difficult or impossible to travel to a lab routinely, particularly in a post-operative condition, putting the safety and health of the patient at further risk postoperatively.
LMWH and unfractionated heparin (UFH) have been proven to be safer than VKAs for long-term management of VTE from the standpoint of prevention of propagation or thrombosis recurrence. With a predictable anticoagulant effect, LMWHs do not require regular lab monitoring, do not require an in-patient hospital stay to initiate treatment, and have a rapid onset and offset action translating to better adaptability when initiating and discontinuing therapy (13). The CLOT study showed that in cancer patients with VTE, long term treatment with a LMWH dalteparin was more effective in reducing the risk of recurrent VTE than treatment with a VKA along with a numerically lower risk of any bleeding (13,15). This study, along with three other randomized clinical trials, form the primary support for current preference for LMWH agents for use in treatment of VTE patients with cancer (16-18).
Aspirin is another potential alternative for chemical VTE prevention, supported primarily in orthopedic literature. A prospective cross-over study of patients undergoing primary total joint arthroplasty (TJA) demonstrated aspirin, both high dose and low dose, is a safe and effective prophylaxis when used in combination with in-hospital mechanical prophylaxis for the prevention of VTE (19). Currently available literature, not specific to the cancer population, shows that low-dose aspirin is not inferior to high dose aspirin for VTE prophylaxis in patients undergoing total joint arthroplasty (19). Very few studies address the use of aspirin for VTE prevention specifically in the cancer population, but one small retrospective review of patients undergoing surgery for a primary malignant soft-tissue or bone tumor or metastatic carcinoma, demonstrated that aspirin may be effective at preventing VTE in patients undergoing orthopaedic oncologic surgery. Research with larger numbers and ideally a more advanced study design should be conducted for further support (20).
More recently, attention has turned to the use of direct oral anticoagulants (DOACs) in patients with cancer undergoing surgery. In non-cancer patients, dabigatran and edoxaban are as effective as VKAs at VTE prevention and are associated with a lower risk of major bleeding (21). The 2016 American College of Chest Physicians recommends DOACs such as rivaroxaban and apixaban over VKAs for treatment of DVT and PE (21). DOACs have a rapid onset of action and predictable pharmacokinetic profile thus avoiding an inpatient initiation of treatment, delay in anticoagulant effects and the necessity for continuous lab monitoring (21). In addition, administration of an oral agent is far more convenient than intravenous infusion or a subcutaneous injection as observed in LMWHs.
Initial support for use of DOACs in patients with cancer comes primarily from two clinical trials, Hokusai-VTE Cancer and Anticoagulation Therapy in Selected Cancer Patients at Risk of Recurrence of Venous Thromboembolism (SELECT-D) (22,23). The Hokusai-VTE Cancer study looked at edoxaban versus dalteparin treatment, and found edoxaban to be statistically noninferior to dalteparin for the composite outcome of recurrent VTE or major bleeding. Absolute rate of recurrence of DVT was lower than dalteparin, but absolute risk of major bleeding was statistically higher, which in subgroup analysis was shown to be only in patients with gastrointestinal cancer. The SELECT-D study evaluated rivaroxaban versus dalteparin. The study demonstrated that rivaroxaban treatment was associated with a lower rate of VTE recurrence, equivalent rate of major bleeding, again primarily in gastrointestinal cancers, and numerically higher rate of clinically relevant non-major bleeding compared to dalteparin treatment.
Regarding orthopedic surgery specifically, apixaban is currently approved for thromboprophylaxis treatment in both total knee replacement surgery and total hip replacement surgery (24). Adequate study has not been performed to determine if there is a benefit or drawback to using these agents versus other agents for prevention of VTE. Initial data from small retrospective series report conflicting evidence regarding whether use of DOACs preoperatively may cause delay of surgery. However, there is currently no specific antidote to reverse its anticoagulant effect (24). Additionally, in regards to post-operative resumption of apixaban, there are no definitive recommendations (24). For low-risk procedures, apixaban may be resumed within 24 h, for high-risk procedures apixaban may be resumed within 48–72 h and for patients unable to take oral medications postoperatively, enoxaparin 40 mg once daily may be used for interim prophylaxis (24). In addition to inconsistencies in guidelines, access to DOACs may prove challenging. DOACs for venous thromboprophylaxis are notoriously more expensive and therefore a certain subset of patients may have limited access (21). Thus, the selection and administration of anticoagulants to patients with cancer undergoing surgery, both established risk factors, must be carefully considered (Table 1).
Table 1
| Prophylaxis method | Type | Advantages | Disadvantages |
|---|---|---|---|
| Mechanical | Sequential Compression Device (SCD) | Low cost, no risk | None |
| Compression stockings | Low cost, no risk | None | |
| Chemical | Vitamin K antagonists (VKA) | Low cost; easily reversible | Interaction with other medications; increased risk for bleeding; delayed onset of anticoagulation; increased length hospital stay; continuous lab monitoring |
| Low molecular weight heparin (LMWH) | Rapid onset; safer than VKA; no lab monitoring; no prolonged inpatient stay | Administered via injection; limited use in renal Insufficiency; risk for HIT | |
| Unfractionated heparin | Rapid onset; safer than VKA | IV administration; risk of HIT; frequent lab monitoring | |
| Aspirin | Low cost; low risk for bleeding | Administered with use of SCD’s | |
| Direct Oral Anticoagulants (DOAC) | Low risk for bleeding; rapid onset; predictable pharmacokinetics; no lab monitoring; no prolonged inpatient stay; oral administration | Difficult reversal; expensive |
Bleeding complications
In orthopedic surgery in particular, a balance must be struck to effectively prevent VTE and avoid undesired wound complications (25,26). The morbidity of wound complications in orthopedic surgery is severe, in many cases requiring removal of orthopedic implants, sometimes without hope of future reimplantation (27). In an attempt to examine how to achieve this balance, a retrospective study examining the high risk of VTE after surgery for long bone metastases, Groot et al. examined the relationship between wound complication rates and chemoprophylaxis (28). Patients were chemically anticoagulated postoperatively with LMWH (358 of 682 patients comprising 52%), no anticoagulant (113, 17%), warfarin (129, 19%), aspirin (66, 10%) and subcutaneous heparin (16, 2%). Notably, compression stockings and compression devices were not included as variables because they were routinely used at the hospital in which the study was conducted.
Overall, 6% (44 of 682) of patients had symptomatic VTE, 22 patients sustained a DVT and 22 patients developed a PE (28). But most significantly, no association was found between wound complications and the use of chemoprophylaxis (P=0.252). Of the 682 patients followed, 9 underwent revision surgery for deep infection, 3 for deep, large hematoma, and 1 large retroperitoneal hematoma (28).
Similarly, Shallop et. al determined no correlation between a specific anticoagulant and rate of wound complications (8). In this study evaluating patients undergoing intramedullary nailing for metastatic bone lesions, the data also reflected no correlation between a specific anticoagulant and rate of wound complication (8). Moreover, none of the patients with wound complications experienced VTE (8). In the patients followed, a total of 11 operations (3.3%) were classified as an infection or “other wound complications” (8). However, the authors note that it is possible that this study may have been underpowered to detect a true difference. The authors also note that the lower incidence of wound complications in patients undergoing intramedullary nailing may be attributable to the limited nature of the incisions in comparison to arthroplasty, and that in many cases treatment of wound complications with intramedullary nailing cases can be treated without implant removal. Therefore, in this population, more aggressive AC may be acceptable when considering wound risks (8).
Risk stratification
In patients with cancer, lowered blood counts, chemotherapy, drug interactions, renal impairment and hepatic involvement with metastases all contribute to a higher risk of bleeding complications (14). Influenced by factors such as cancer type, chemotherapy, surgical intervention and thrombocytopenia (29), the risk of major bleeding increases significantly with metastatic disease and immobility greater than or equal to four days (29). Currently, there is no bleeding score to assess the risk of patients with cancer. Thus, an individualized approach of risk assessment is recommended (29). However, the implementation of a more standard risk stratification system, inclusive of patient’s comorbidities, would potentially decrease the risk of VTE by making the process of selecting and administering anticoagulants happen according to a data-driven protocol.
A study examining the potential to decrease the number of surgically associated thromboembolic events by embedding a risk stratification tool into the electronic medical record (EMR) system using Epic Systems Corporation (Epic) software proved the effectiveness of such a risk stratification system (30). The site of the study used a tool in Epic to link a patient’s risk level to specific VTE prophylaxis order sets. Risk factors for stratification included many factors including a history of cancer and undergoing a major operation. When providers were compliant, consistency and accuracy in AC selection improved thus decreasing VTE rates (30). A similar study was conducted examining the benefits of a risk stratification system for the occurrence of a PE in patients undergoing total joint arthroplasty (TJA). In this study, a point scoring system was introduced to estimate the risk for PE (31). As a result, the point scoring system was able to help predict the risk for PE after TJA (31). Findings in other studies have suggested other risk factors could be added for consideration when building a stratification system. Due to correlation between primary lung histology and increased risk of VTE as well as post-operative radiation and an increased risk of VTE, Shallop et al. suggests AC protocols should be adjusted to a patient’s primary disease (8). Similarly, Ratasvuori et al. determined intraoperative oxygen saturation drop, pulmonary metastases and intramedullary nailing were all independent risk factors for VTE (14). These factors, as well as many others, could be built into a risk stratification system to help tailor anticoagulant regimens even more specifically to patients with MBD and other comorbidities thus effectively decreasing the occurrence of VTE.
Timing of prophylactic treatment
In an early retrospective study, De la Garza Ramos et al. investigated the effect of timing of initiation of prophylactic AC on the incidence of VTE after surgery for metastatic tumors of the spine, where bleeding complications could be disastrous (32). Out of 65 patients included for the purpose of the study, 36/65 (56%) received prophylactic AC in addition to mechanical prophylaxis between days 1 and 3 after surgical intervention. With only one case of an epidural hematoma (1.5%), the results of the study showed a significant reduction in the risk of DVT and PE occurring within 30 days for patients undergoing surgery for metastatic tumors of the spine (32). Although further research was encouraged, the findings preliminarily showed that “early” prophylactic AC post-operatively is reasonably safe and potentially decreased the risk of VTE events (32). In a similar but more recent study examining the risk for VTE in patients undergoing surgery for metastatic disease of the spine, all chemoprophylaxis was started within 48 hours after surgery. Contrary to the previous study, in this population, a significant 11% of patients experienced symptomatic VTE (6% PE and 6% DVT) and overall 10% of patients developed a wound complication including 1.1% spinal epidural hematomas (25).
In patients undergoing surgery for non-spinal skeletal metastasis largely due to pathological fractures, a significant 10% of patients experienced symptomatic VTE with an overall incidence of 3.3% fatal PE (14). As a result, the author implored greater collaboration for appropriate risk stratification of patients between hematologists and orthopaedic oncology surgeons (14), and with respect to timing of AC, raises the issue of preoperative anticoagulation. Administration of preoperative anticoagulants was previously feared for high risk of infection, increased bleeding and other complications. However, in a single institution prospective study evaluating the safety and efficacy of adding pre and post AC in major non-orthopedic surgery, Selby et al. found no significant increase in bleeding complication between those that received anticoagulants preoperatively and those that did not (33). Additionally, the study showed that in patients undergoing major cancer surgery, a single dose of a preoperative chemoprophylaxis did not increase bleeding or blood transfusions (33). When comparing those that did not receive preoperative chemoprophylaxis, and those that did in the form of UFH (5,000 U) or LMWH (40 mg enoxaparin) within 2 h prior to the operation, the cohort that did receive preoperative chemoprophylaxis had significantly lower rates of bleeding complications, blood transfusions, DVT and PE (33). Thus, the benefits of preoperative chemoprophylaxis at preventing the occurrence of VTE seem to outweigh the minimal risk of excessive bleeding. Further study will need to be done to evaluate this strategy in the MBD surgical population.
Conclusions
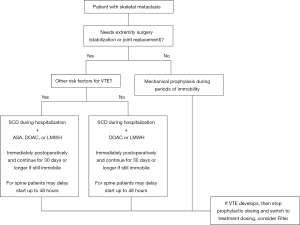
With a reported 84% of patients with breast or prostate cancer displaying skeletal deposits at post mortem (7), the impact of metastatic skeletal disease is staggering. Metastatic skeletal disease, commonly requiring orthopaedic surgery, puts patients a high risk of VTE. As such, a variety of chemical anticoagulants are effective, particularly when administered sooner rather than later, at reducing the risk of VTE. Careful choice of anticoagulant and timing of administration must be made in order to avoid bleeding complications (Figure 1). A risk stratification system to determine which chemical prophylaxis to administered could be beneficial in both reducing the occurrence of VTE and decreasing associated wound complications or mortality. Further study should be conducted to tailor chemical prophylaxes recommendations to this largely affected population and effectively reduce the occurrence of VTE.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rui Yang) for the series “Bone Metastasis” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-107/coif). The series “Bone Metastasis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Falanga A, Marchetti M, Russo L. The mechanisms of cancer-associated thrombosis. Thromb Res 2015;135:S8-11. [Crossref] [PubMed]
- Abdol Razak NB, Jones G, Bhandari M, et al. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers (Basel) 2018;10:380. [Crossref] [PubMed]
- Sud R, Khorana AA. Cancer-associated thrombosis: risk factors, candidate biomarkers and a risk model. Thromb Res 2009;123:S18-21. [Crossref] [PubMed]
- Connolly GC, Khorana AA. Emerging risk stratification approaches to cancer-associated thrombosis: risk factors, biomarkers and a risk score. Thromb Res 2010;125:S1-7. [Crossref] [PubMed]
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003;107:I17-21. [Crossref] [PubMed]
- Geerts WH, Jay RM, Code KI, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med 1996;335:701-7. [Crossref] [PubMed]
- Eastley N, Newey M, Ashford RU. Skeletal metastases - the role of the orthopaedic and spinal surgeon. Surg Oncol 2012;21:216-22. [Crossref] [PubMed]
- Shallop B, Starks A, Greenbaum S, et al. Thromboembolism After Intramedullary Nailing for Metastatic Bone Lesions. J Bone Joint Surg Am 2015;97:1503-11. [Crossref] [PubMed]
- Aneja A, Jiang JJ, Cohen-Rosenblum A, et al. Thromboembolic Disease in Patients with Metastatic Femoral Lesions: A Comparison Between Prophylactic Fixation and Fracture Fixation. J Bone Joint Surg Am 2017;99:315-23. [Crossref] [PubMed]
- Lin PP, Graham D, Hann LE, et al. Deep venous thrombosis after orthopedic surgery in adult cancer patients. J Surg Oncol 1998;68:41-7. [Crossref] [PubMed]
- Woolson ST, Watt JM. Intermittent pneumatic compression to prevent proximal deep venous thrombosis during and after total hip replacement. A prospective, randomized study of compression alone, compression and aspirin, and compression and low-dose warfarin. J Bone Joint Surg Am 1991;73:507-12. [Crossref] [PubMed]
- Yamaguchi T, Matsumine A, Niimi R, et al. Deep-vein thrombosis after resection of musculoskeletal tumours of the lower limb. Bone Joint J 2013;95-B:1280-4. [Crossref] [PubMed]
- Falanga A, Zacharski L. Deep vein thrombosis in cancer: the scale of the problem and approaches to management. Ann Oncol 2005;16:696-701. [Crossref] [PubMed]
- Ratasvuori M, Lassila R, Laitinen M. Venous thromboembolism after surgical treatment of non-spinal skeletal metastases - An underdiagnosed complication. Thromb Res 2016;141:124-8. [Crossref] [PubMed]
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53. [Crossref] [PubMed]
- Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial. JAMA 2015;314:677-86. [Crossref] [PubMed]
- Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006;119:1062-72. [Crossref] [PubMed]
- Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med 2002;162:1729-35. [Crossref] [PubMed]
- Parvizi J, Huang R, Restrepo C, et al. Low-Dose Aspirin Is Effective Chemoprophylaxis Against Clinically Important Venous Thromboembolism Following Total Joint Arthroplasty: A Preliminary Analysis. J Bone Joint Surg Am 2017;99:91-8. [Crossref] [PubMed]
- Mendez GM, Patel YM, Ricketti DA, et al. Aspirin for Prophylaxis Against Venous Thromboembolism After Orthopaedic Oncologic Surgery. J Bone Joint Surg Am 2017;99:2004-10. [Crossref] [PubMed]
- Tritschler T, Kraaijpoel N, Le Gal G, et al. Venous Thromboembolism: Advances in Diagnosis and Treatment. JAMA 2018;320:1583-94. [Crossref] [PubMed]
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med 2018;378:615-24. [Crossref] [PubMed]
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol 2018;36:2017-23. [Crossref] [PubMed]
- Zalpour A, Oo TH. Clinical utility of apixaban in the prevention and treatment of venous thromboembolism: current evidence. Drug Des Devel Ther 2014;8:2181-91. [PubMed]
- Groot OQ, Ogink PT, Paulino Pereira NR, et al. High Risk of Symptomatic Venous Thromboembolism After Surgery for Spine Metastatic Bone Lesions: A Retrospective Study. Clin Orthop Relat Res 2019;477:1674-86. [Crossref] [PubMed]
- Parvizi J, Ghanem E, Joshi A, et al. Does "excessive" anticoagulation predispose to periprosthetic infection? J Arthroplasty 2007;22:24-8. [Crossref] [PubMed]
- Zmistowski B, Karam JA, Durinka JB, et al. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am 2013;95:2177-84. [Crossref] [PubMed]
- Groot OQ, Ogink PT, Janssen SJ, et al. High Risk of Venous Thromboembolism After Surgery for Long Bone Metastases: A Retrospective Study of 682 Patients. Clin Orthop Relat Res 2018;476:2052-61. [Crossref] [PubMed]
- Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res 2014;133:S49-55. [Crossref] [PubMed]
- Turrentine FE, Sohn MW, Wilson SL, et al. Fewer thromboembolic events after implementation of a venous thromboembolism risk stratification tool. J Surg Res 2018;225:148-56. [Crossref] [PubMed]
- Bohl DD, Maltenfort MG, Huang R, et al. Development and Validation of a Risk Stratification System for Pulmonary Embolism After Elective Primary Total Joint Arthroplasty. J Arthroplasty 2016;31:187-91. [Crossref] [PubMed]
- De la Garza Ramos R, Longo M, Gelfand Y, et al. Timing of Prophylactic Anticoagulation and Its Effect on Thromboembolic Events After Surgery for Metastatic Tumors of the Spine. Spine (Phila Pa 1976) 2019;44:E650-5. [Crossref] [PubMed]
- Selby LV, Sovel M, Sjoberg DD, et al. Preoperative Chemoprophylaxis is Safe in Major Oncology Operations and Effective at Preventing Venous Thromboembolism. J Am Coll Surg 2016;222:129-37. [Crossref] [PubMed]
Cite this article as: Donahue A, Sobol KR, Abraham JA. Venous thromboembolism in musculoskeletal oncology surgery. Ann Joint 2022;7:39.


