
Skeletal metastatic disease of the acetabulum: historical and evolving techniques for management
Introduction
The incidence of new cancer diagnoses has remained stable in the United States, while mortality from cancer has decreased slightly over the past decade (1). This means more patients are living with a cancer diagnosis in the United States than ever before. The number of patients who will develop skeletal metastatic disease annually in the United States is more difficult to predict, but ranges from 280,00–400,00 patients (2,3). The incidence of skeletal related events, as defined by patients with metastatic skeletal disease who develop pathologic fracture, spinal cord compression or need surgery or radiation for their metastatic disease, has been detailed in the placebo arms of a number of trials evaluating the efficacy of antiresorptive therapies. For instance, in trials randomizing patients to placebo versus bisphosphonate therapy, pathologic fractures were identified in 52% of patients with metastatic breast cancer in the placebo arm (4), as compared to 25% of patients with metastatic prostate carcinoma (5), or 22% of patients with metastatic lung carcinoma (6). The economic burden associated with the treatment of patients with metastatic skeletal disease was estimated to be 12.6 billion dollars in 2004. That number most certainly has grown over time (7).
Bone represents the third most common organ system to be involved in the distant spread of a carcinoma (3). The distribution of metastatic disease in the skeleton has been well characterized. Most metastases will involve the axial skeleton, with the spine, ribs, sternum and pelvis representing the four most common sites of disease (8). Any orthopaedic surgeon who regularly treats metastatic skeletal disease will most certainly encounter challenges from patients presenting with metastatic acetabular disease.
Classification systems for metastatic disease of the acetabulum
Enneking in 1978 described one of the first classification systems used for the surgical management of malignancies of the pelvis (9). His classification system was simple, but is still widely used. He divided the pelvis into three zones: zone one involves the pelvis, between the acetabulum and sacroiliac joint, zone two involves the acetabulum itself, and zone three involving structures medial to the acetabulum. Harrington in 1981 published his results on the management of acetabular metastatic disease, and proposed a classification system (10). He divided acetabular involvement into type 1 lesions where the acetabular columns and walls are preserved, type 2 lesions where the medial wall and quadrilateral surface of the pelvis are disrupted, and type 3 lesions where the roof and superior rim of the acetabulum are disrupted, often with large iliac wing lesions involved. The Metastatic Acetabular Classification (MAC) classifies acetabular metastases into four types, including involvement of the acetabular dome (Type 1), involvement of the medial wall (Type 2), involvement of a single column (Type 3), involvement of both columns (Type 4) (11,12). Paprosky in 1994 defined acetabular defects in the setting of revision total hip arthroplasty (13). While his classification system was not specific to metastatic skeletal disease, it is similar to the Harrington and MAC systems, and offers specific reconstruction techniques for specific acetabular defects.
Importantly, all of these classification systems highlight the same basic principles. The first is that adequate pre-operative planning is essential. As much information as possible, often utilizing MRI and CT imaging, should be gathered about the size and location of bony defects about the acetabulum. Second, an appropriate approach and exposure is needed and can be successfully gained with good preoperative planning. Third, efforts should be made to remove as much pathologic bone as possible. Fourth, medial wall defects are prone to failure by protrusion, and implants preventing that mode of failure should be selected. Fifth, adequate bone stock for implant and cement fixation is needed beyond the acetabular defect.
Treatment options
The treatment options for patients presenting with metastatic skeletal disease involving the acetabulum have evolved significantly. The use of ablative therapies, minimally invasive procedures for cementation and hardware placement, and surgical hip reconstruction procedures will be reviewed.
A multidisciplinary care team
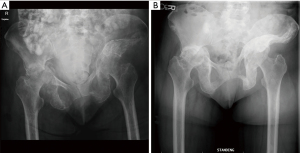
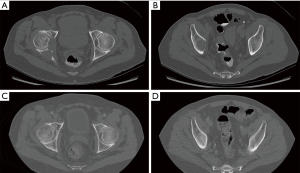
Many patients present with well-contained, small acetabular lesions that do not require any site-specific therapy. It is important to understand that not all patients will require invasive procedures. The value of a multidisciplinary team of providers with expertise in treating skeletal metastatic disease cannot be overstated (14,15). Specifically, teams that involve medical oncologists, radiation oncologists, orthopaedic oncologists, interventional radiology and palliative care have been organized and demonstrate improved patient care (14). These teams can best assess treatment options for patients on an individual basis, emphasizing patient prognosis and goals of care. Often, the least invasive therapies are offered first to patients, saving surgical reconstruction options for evolving, significantly symptomatic disease. Even symptomatic patients with lesions involving the weight bearing dome, medial wall, or column can be treated initially with protected weight bearing, systemic therapy and radiation therapy with good results (Figures 1,2). The decision-making in these cases can be quite complex, and a multidisciplinary team proves very helpful.
Ablative therapies
A subset of patients presenting with metastatic disease to the skeleton may be candidates for an ablative procedure, often using percutaneous image guided techniques. These patients may have painful metastases with limited impact on the articular surface of the acetabulum. Often, they have failed prior therapies such as systemic therapy, antiresorptive therapy or radiation. Many patients wish to avoid surgery, or are poor surgical candidates, and may see benefit from a less invasive ablative treatment. The hope is that a direct ablation of metastatic cancer will decrease pain, and with time, remodeling of the bone in the area can provide structural support and prevent fracture.
The two best studied techniques for image guided ablation of skeletal metastases are radiofrequency ablation (RFA) and cryoablation. RFA probes heat tissue to approximately 90 degrees Celsius and on average generate a zone of ablation measuring 4 cm in diameter, although techniques have been described to control the zone of ablation using multiple probes, or simultaneous cooling techniques (16,17). RFA carries the disadvantages of an inability to simultaneously image the zone of ablation with conventional CT or US imaging, and the lack of adequate penetration of heat beyond cortical bone (less effective for metastatic lesions with associated soft tissue components). Cryoablation is gaining more favor in recent studies as the optimal technique for ablation of skeletal metastases (18-20). Cryoablation works to kill neoplastic cells via rapid cooling and thawing, with freezing temperatures reaching −40 to −60 degrees Celsius. Multiple probes can be utilized to accommodate larger metastases. The “ice ball” generated with cryoablation can be visualized with CT imaging, allowing for real time monitoring of the zone of ablation, and cryoablation techniques are more successful at penetrating cortical bone. Multiple reports have demonstrated effective RFA and cryoablation of small to large metastatic lesions, ranging from 1–18 cm in size (18-20). Thacker et al. (18) performed an evaluation of RFA versus cryoablation for painful metastatic disease to bone, including 31 metastases involving the pelvis and acetabulum. They found that patients treated with cryoablation had lower immediate post-procedure pain scores and shorter post procedure length of stay. Gardner et al. reported on 6 patients with metastatic renal cell cancer to the acetabulum successfully treated with cryoablation therapy (20). Bauones treated three patients with painful acetabular metastases using thermal ablation, and temperature monitoring in an effort to protect the acetabular cartilage (17). The MOTION multicenter trial is a prospective multicenter trial that recently published results on the safety and efficacy of cryoablation used for painful skeletal metastases, including nineteen patient with pelvic and acetabular involvement (19). The MOTION trial authors found a decrease in pain scores and an increase in quality of life measures that persisted up to 24 weeks post treatment. Of note, patient’s mean pain scores dropped from 7.3 on a ten-point scale to 3.7, suggesting an improvement, but incomplete palliation of pain (19). There are a number of issues that should be considered when planning an ablation procedure including the size of the metastatic lesion, proximity to vital structures, proximity to the joint surface and pathologic fracture risk. For these reasons, an experienced interventional radiologist should be involved and there is benefit to discussing these cases in a multidisciplinary fashion.
Percutaneous structural augmentation
Acetabular metastatic disease is often accompanied by significant bone loss, and structural instability of the acetabulum and surrounding pelvis. In these cases, ablation therapies alone may not be sufficient to restore structural support. A number of techniques have been described that combine percutaneous ablation therapies with cementation and percutaneous screw placement. Wallace et al. demonstrated success with an image guided technique for RFA and percutaneous cement injection for contained acetabular defects in metastatic disease in 12 patients (21). Follow up was only to a median of 62 days, but no immediate post-procedure complications were noted. In a study of 11 patients who were treated with percutaneous cementation of acetabular metastases, median follow up was 26.4 months, with two of the ten patients requiring further intervention for evolving disease or symptoms (22). Powell et al. present two cases of percutaneous cement and screw placement for very large periacetabular metastatic tumors with reported pain relief beyond 1 year (23). Yang et al. recently published a series of patients with metastatic acetabular disease treated with percutaneous screw placement alone (24). In their series, three cannulated screws, one in the anterior column, one in the posterior column and a third in a “trans-columnar” fashion were placed percutaneously without cement augmentation. They demonstrated good pain relief in the majority of patients. Four patients progressed to needing an open reconstruction with hip arthroplasty, in which case the previously placed screws were maintained to assist with augmentation.
Surgical hip reconstruction
Patients can present with very large areas of periacetabular metastatic disease at the time of initial cancer diagnosis, or disease that has been refractory to previous treatments. Often this results in pathologic fracture of the acetabulum. Pain and dysfunction for these patients can be severe. These patients may be candidates for surgical reconstruction, usually with variations of total hip arthroplasty. An approach for total hip arthroplasty allows for extensive exposure of the acetabulum, with treatment of metastatic tumors and bone loss through the joint itself. The metastatic tumor can be curetted from the bone, and surgical adjuvants can be applied. Pelvic reconstruction is then accomplished with a hip arthroplasty, using a number of techniques that will be reviewed here.
Harrington published his series of 58 patient treated with hip arthroplasty for pathologic fractures of the acetabulum in metastatic disease (10). He described three different patterns of disease that were managed with cemented acetabular reconstruction using Steinman pins or anti-protrusio cages. In general, he reported relatively good short-term outcomes with these techniques. However, only 45% of patients were ambulatory 2 years post-surgery, and five of his reconstructions failed secondary to advancing metastatic disease.
The Harrington technique has evolved over time with variable outcomes reported in the literature. Marco et al. reviewed the outcomes of 54 patients treated for metastatic acetabular disease over the course of 10 years at Memorial Sloan Kettering (12). The patients were treated with either cemented hip arthroplasty alone, a modified Harrington reconstruction with retrograde screws, or a modified Harrington reconstruction with antegrade screws or Steinman pins. The majority of patients had an anti-protrusio acetabular cup placed. The authors were the first to describe a very useful triangulation guide for pin insertion, which is positioned in the acetabulum and allows for a more accurate antegrade screw or pin positioning. A tibial drilling guide found in an ACL reconstruction set can work well for most patients as well. The authors reported a 22% early complication rate, with less than half of patients surviving more than 1 year. There were five reported fixation failures in surviving patients at 12 months. Pain and function improved in most patients, and the authors argued their reconstruction techniques are justified as a palliative procedure, despite complications and overall low survival.
A number of other studies have demonstrated similar success and complication rates with a variety of different reconstruction techniques. Tillman et al. describe outcomes in 19 patients treated with three antegrade Steinman pins and a cemented liner (25). Clayer et al. (26) and Rowell et al. (27) both described outcomes with the use of an anti-protrusio cage and cement construct.
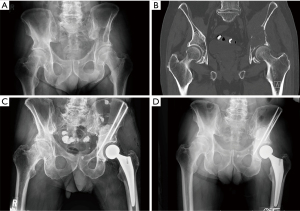
Despite the potential complications that can be seen with a Harrington reconstruction, the technique of total hip arthroplasty using cemented implants, augmented with pins or anti-protrusio cages, is still a warranted a valuable construction option for patient with large periacetabular defects from metastatic disease (Figure 3).
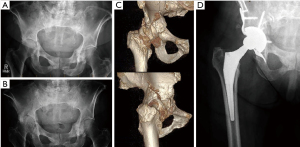
With improved systemic therapy options, more patients with skeletal metastatic disease are living longer. There is growing interest in the use of techniques that may allow for more durability of reconstruction as compared to cemented Harrington reconstructions. Most notably, the use of tantalum metal with high porosity has shown promise in the setting of periacetabular metastatic disease, or in patients who have a history of pelvic irradiation (28) (Figure 4). Khan et al. reported on 20 patients treated with porous tantalum acetabular components and total hip arthroplasty at the Mayo Clinic (29). A combination of tantalum metal augments, acetabular shells and anti-protrusio cages were used. No cement, outside of a small bead of cement between the augment and shell was used. More than half of their patients had died less than 2 years post-surgery, but in the remaining patients, there was no implant failure.
Another evolving prosthetic design is the pedestal cup, or “ice cream cone” acetabular prosthesis. This prosthesis utilizes a press fit technique to place a hydroxyapatite coated conical stem into the sciatic buttress if the ilium. Lowe et al. reported on 24 patients with periacetabular metastatic disease treated with a pedestal cup prosthesis (30). They found a 22% complication rate at a mean follow-up of 36 months, with dislocation and deep infection occurring in 8% and 12% of patients respectively. They highlight decreased complications in cases using intraoperative navigation, and an overall survival rate of the implant of 90% at 5 years. A number of other studies have demonstrated success with the use of this implant design in periacetabular metastatic disease (31,32).
Newer technologies allow for custom-made implants for patients with periacetabular bone loss secondary to metastatic disease. This provides a powerful tool for the surgical team. Pre-operative imaging can be used to plan for and build implants the match a patient’s bone loss. These implants can allow for bony ingrowth, screw fixation and/or stemmed fixation, potentially allowing for immediate bony stability and limited dependence on cement. Custom implants come with the drawbacks of time needed for manufacture (5–8 weeks on average) and significant monetary cost. Ji et al. recently published their results with custom made 3D printed modular hemipelvic endoprostheses in a series of 80 patients, including 16 patients with metastatic disease to the acetabulum (33). They reported no cases of aseptic loosening in their series with an average follow up of 33 months.
Comparing techniques
Unfortunately, the majority of publications detailing reconstruction techniques and outcomes for metastatic acetabular disease are of poor quality. There are few studies that compare techniques. Colman et al. compared outcomes of patients treated with percutaneous cement acetabuloplasty versus cemented total hip arthroplasty using Steinman pin columnar reconstruction and anti-protrusio cages in 28 patients (34). They found lower complication rates in the acetabuloplasty group, but overall better pain reduction and better functional scores in the surgery group. Houdek et al. recently compared reconstructions with tantalum acetabular implants to those using the modified Harrington technique with cemented reconstructions (35). They compared 78 patients treated with the Harrington reconstruction at one institution to a group of 37 patients treated with tantalum acetabular reconstructions at a separate institution. They found a significantly lower all cause revision rate in the tantalum group, with no cases of acetabular loosening, as opposed to five cases of implant loosening in the Harrington group. It should be noted the mean patient survival was only 34% at 2 years, limiting the evaluation of long-term durability in both groups. A systematic review published in 2018 evaluated the outcomes of 1,700 patients pooled from 57 studies and treated with a number of different reconstruction techniques (36). These included patients with primary bone tumors as well as metastatic disease. Seven reconstruction techniques were compared, including the Harrington technique, reconstructions using tantalum implants, and reconstructions using custom made implants. They identified an overall complication rate of 50% after these complex procedures, but suggested better early radiographic and functional outcomes with tantalum metal reconstructions and custom-made implants.
Conclusions
While a number of different techniques, using a variety of different implants and technologies have been described for the treatment of acetabular insufficiency in the setting of metastatic disease, there most certainly is no consensus on which technique works best. Unfortunately, the problem in itself is diverse. Different tumor types will respond differently to adjuvant therapies. The influence of radiation therapy or prior ablation procedures on the durability of a hip reconstruction is not fully understood. There is likely a significant amount of variability on the success of a hip reconstruction based on the size and location of the treated metastatic disease alone. Considerations should also be given to health care cost for the palliative treatment of skeletal metastatic disease. There are no reliable publications comparing the costs of the treatment approaches used for metastatic disease to the acetabulum. For all of these reasons, the orthopaedic surgeon managing metastatic acetabular disease must be aware of their patient’s goals, the opinions provided by other providers in a multidisciplinary team, and the options at their fingertips in the operating room. Systemic therapy alone may work best for one patient, while radiation or an ablation is better for the next, or a surgical hip reconstruction for the third.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rui Yang) for the series “Bone Metastasis” published in Annals of Joint. The article has undergone external peer review.
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-117/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-117/coif). The series “Bone Metastasis” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol 2012;4:87-93. [PubMed]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584-93. [Crossref] [PubMed]
- Lipton A. Bisphosphonates and breast carcinoma: present and future. Cancer 2000;88:3033-7. [Crossref] [PubMed]
- Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004;96:879-82. [Crossref] [PubMed]
- Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer 2004;100:2613-21. [Crossref] [PubMed]
- Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer 2007;109:2334-42. [Crossref] [PubMed]
- Kakhki VR, Anvari K, Sadeghi R, et al. Pattern and distribution of bone metastases in common malignant tumors. Nucl Med Rev Cent East Eur 2013;16:66-9. [Crossref] [PubMed]
- Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am 1978;60:731-46. [Crossref] [PubMed]
- Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am 1981;63:653-64. [Crossref] [PubMed]
- Issack PS, Kotwal SY, Lane JM. Management of metastatic bone disease of the acetabulum. J Am Acad Orthop Surg 2013;21:685-95. [Crossref] [PubMed]
- Marco RA, Sheth DS, Boland PJ, et al. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am 2000;82:642-51. [Crossref] [PubMed]
- Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty 1994;9:33-44. [Crossref] [PubMed]
- Ibrahim T, Flamini E, Fabbri L, et al. Multidisciplinary approach to the treatment of bone metastases: Osteo-Oncology Center, a new organizational model. Tumori 2009;95:291-7. [Crossref] [PubMed]
- Kimura T. Multidisciplinary approach for bone metastasis: a review. Cancers (Basel) 2018;10:156. [Crossref] [PubMed]
- Ahmed O, Feinberg N, Lea WB. Interventional techniques for the ablation and augmentation of extraspinal lytic bone metastases. Semin Intervent Radiol 2019;36:221-8. [Crossref] [PubMed]
- Bauones S, Garnon J, Chari B, et al. Protection of the proximal articular cartilage during percutaneous thermal ablation of acetabular metastasis using temperature monitoring. Cardiovasc Intervent Radiol 2018;41:163-9. [Crossref] [PubMed]
- Thacker PG, Callstrom MR, Curry TB, et al. Palliation of painful metastatic disease involving bone with imaging-guided treatment: comparison of patients' immediate response to radiofrequency ablation and cryoablation. AJR Am J Roentgenol 2011;197:510-5. [Crossref] [PubMed]
- Jennings JW, Prologo JD, Garnon J, et al. Cryoablation for palliation of painful bone metastases: the MOTION multicenter study. Radiol Imaging Cancer 2021;3:e200101. [Crossref] [PubMed]
- Gardner CS, Ensor JE, Ahrar K, et al. Cryoablation of bone metastases from renal cell carcinoma for local tumor control. J Bone Joint Surg Am 2017;99:1916-26. [Crossref] [PubMed]
- Wallace AN, Huang AJ, Vaswani D, et al. Combination acetabular radiofrequency ablation and cementoplasty using a navigational radiofrequency ablation device and ultrahigh viscosity cement: technical note. Skeletal Radiol 2016;45:401-5. [Crossref] [PubMed]
- Durfee RA, Sabo SA, Letson GD, et al. Percutaneous acetabuloplasty for metastatic lesions to the pelvis. Orthopedics 2017;40:e170-5. [Crossref] [PubMed]
- Powell DK, Ardestani A. Percutaneous screw-reinforced cement osteoplasty for palliation of postremission pain in larger lytic sacro-acetabular iliac cavities. Radiol Case Rep 2019;14:1093-9. [Crossref] [PubMed]
- Yang R, Goch A, Murphy D, et al. A novel tripod percutaneous reconstruction technique in periacetabular lesions caused by metastatic cancer. J Bone Joint Surg Am 2020;102:592-9. [Crossref] [PubMed]
- Tillman RM, Myers GJ, Abudu AT, et al. The three-pin modified 'Harrington' procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br 2008;90:84-7. [Crossref] [PubMed]
- Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop Relat Res 2010;468:2980-4. [Crossref] [PubMed]
- Rowell P, Lowe M, Sommerville S, et al. Is an acetabular cage and cement fixation sufficiently durable for the treatment of destructive acetabular metastases? Clin Orthop Relat Res 2019;477:1459-65. [Crossref] [PubMed]
- Joglekar SB, Rose PS, Lewallen DG, et al. Tantalum acetabular cups provide secure fixation in THA after pelvic irradiation at minimum 5-year followup. Clin Orthop Relat Res 2012;470:3041-7. [Crossref] [PubMed]
- Khan FA, Rose PS, Yanagisawa M, et al. Surgical technique: Porous tantalum reconstruction for destructive nonprimary periacetabular tumors. Clin Orthop Relat Res 2012;470:594-601. [Crossref] [PubMed]
- Lowe M, Jeys L, Grimer R, et al. Pelvic reconstruction using pedestal endoprosthesis—experience from Europe. Ann Joint 2019;4:34. [Crossref]
- Hipfl C, Stihsen C, Puchner SE, et al. Pelvic reconstruction following resection of malignant bone tumours using a stemmed acetabular pedestal cup. Bone Joint J 2017;99-B:841-8. [Crossref] [PubMed]
- Guzik G. Oncological, surgical and functional results of the treatment of patients after hemipelvectomy due to metastases. BMC Musculoskelet Disord 2018;19:63. [Crossref] [PubMed]
- Ji T, Yang Y, Tang X, et al. 3D-printed modular hemipelvic endoprosthetic reconstruction following periacetabular tumor resection: early results of 80 consecutive cases. J Bone Joint Surg Am 2020;102:1530-41. [Crossref] [PubMed]
- Colman MW, Karim SM, Hirsch JA, et al. Percutaneous acetabuloplasty compared with open reconstruction for extensive periacetabular carcinoma metastases. J Arthroplasty 2015;30:1586-91. [Crossref] [PubMed]
- Houdek MT, Ferguson PC, Abdel MP, et al. Comparison of porous tantalum acetabular implants and Harrington reconstruction for metastatic disease of the acetabulum. J Bone Joint Surg Am 2020;102:1239-47. [Crossref] [PubMed]
- Brown TS, Salib CG, Rose PS, et al. Reconstruction of the hip after resection of periacetabular oncological lesions: a systematic review. Bone Joint J 2018;100-B:22-30. [Crossref] [PubMed]
Cite this article as: Lindsay AD. Skeletal metastatic disease of the acetabulum: historical and evolving techniques for management. Ann Joint 2022;7:27.


