
Cemented-augmented fixation of metastatic humeral lesions without segmental bone loss results in reliable outcomes
Introduction
Cancer is one of the leading causes of death in the United States, with a projected 1,688,780 new cases in 2017 alone (1). With medical and surgical advancement, survival rates have increased for a variety of cancer types. Bone is the third highest location for metastatic disease, following the lungs and liver (2,3). Metastatic bone disease is associated with a rapid decline in life expectancy and quality of life (4). Increasing life expectancies have led to an increase in patients with metastatic bone disease (1,4). As a result of increased life expectancies in this patient population, considerations for surgical management have increased. Initial management of metastatic bone disease includes chemotherapeutics, bisphosphonates, and radiation therapy, with surgery typically reserved for pathologic or impending pathologic fractures (2,5-9).
Goals of operative fixation include decreasing tumor burden, local stabilization, and pain control (8). Surgical fixation of the appendicular skeleton has traditionally included a method of plate or nail stabilization [open reduction internal fixation (ORIF)] following curettage with or without cementation (8). However, disease associated with metaphyseal or epiphyseal fractures have been increasingly managed with hemiarthroplasty or total joint endoprosthesis depending on the level of bone involvement (8). The purpose of this study was to retrospectively report on outcomes of patients managed with either cemented endoprosthetic replacement (EPR) or ORIF and ask the following questions: (I) do complication risks differ following metastatic lesions with endoprosthetic reconstruction versus cemented osteosynthesis? (II) Do functional outcomes differ between methods of reconstruction? (III) Does tumor volume impact outcomes?
Methods
Subjects
The records of 229 consecutive patients treated surgically for metastatic disease from 2005–2018 at our musculoskeletal oncology center were retrospectively reviewed following institutional review board (IRB) approval. Inform consent was waived due to the retrospective nature of this study. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Indications for surgical treatment at the humerus included patients who presented with displaced pathologic fractures and patients with a Mirels score greater than 8 (7).
Treatment considerations
Overall, goals of surgery were to improve pain control, restore function, and minimize risk of re-operation. Patients underwent CT scans pre-operatively to access the extent of bone involvement, as well as for pre-operative planning. There was no distinct numerical measurement regarding cortical thickness or bone loss. However, if there was concern for cortical integrity, particularly at the proximal humerus, EPR was strongly considered. Additionally, the presence of segmental bone loss strongly influenced consideration for reconstruction with EPR.
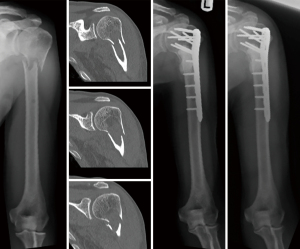
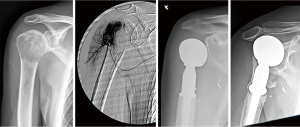
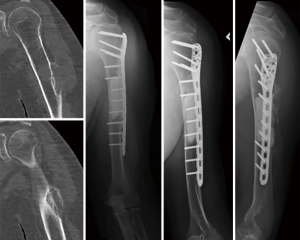
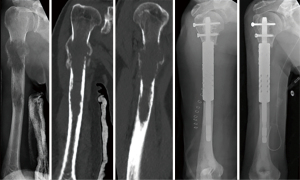
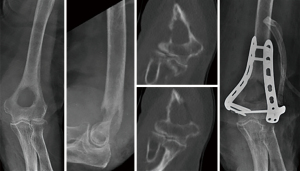
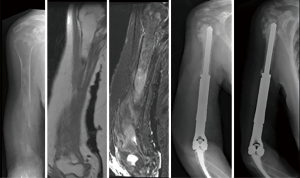
At the proximal humerus, if ORIF was carried out, curettage was performed, followed by fixation with a proximal humeral locking plate, and cementation of residual bone defect. To improve construct strength, screws were placed in cement to augment fixation (Figure 1). In patients with lesions compromising fixation options, EPR was performed typically using a proximal humeral replacement (Figure 2). At the humeral shaft, in patients without segmental cortical destruction, ORIF was typically performed. A similar order of curettage, fixation, cementation, and placement of additional screw fixation in cement was utilized as described above (Figure 3). Patients with segmental cortical destruction, with solitary renal cell carcinoma lesions and projected longer lifespan have traditionally been considered for resection and reconstruction with an intercalary endoprosthesis as previously described (Figure 4) (10). At the distal humerus, ORIF was performed when residual bone stock was amenable to fixation and lesions did not invade the articular surface (Figure 5). In cases where segmental bone destruction was present or invasion of the articular surface occurred, reconstruction was performed with a distal humeral replacement (Figure 6).
Outcome measures
The following characteristics were analyzed between groups: tumor location, tumor volume and circumferential involvement of bone utilizing anterior posterior (AP) and lateral radiographs, Musculoskeletal Tumor Society (MSTS) functional outcome scores, and complications. MSTS scores were calculated for patients with at least 3 months of clinical follow-up.
Statistical analysis
Non-parametric statistical analysis of categorical information was performed using a chi-square test, unless an expected value was less than five, in which case Fischer’s exact test was utilized. Odds ratios were generated with computation of confidence intervals utilizing the Baptista-Pike method. Non-parametric analysis of continuous variables was performed using a Mann-Whitney U test. All analyses were performed using GraphPad Prism version 7.00 for Mac OS X (GraphPad Software, La Jolla, California, USA, www.graphpad.com). In all tests, significance was set at P<0.05.
Results
Sixty patients were identified who were treated surgically at the proximal humerus (n=21), humeral shaft (n=29), or distal humerus (n=10). Forty-nine (82%) patients presented with pathologic fracture. The remaining eleven patients had a mean Mirels score of 9.5. Pathologic diagnoses included renal cell carcinoma (n=16), multiple myeloma (n=11), breast (n=10), lung (n=10), colon (n=3), cervical (n=2), skin (n=2), liver (n=1) prostate (n=1), urothelial (n=1), and thyroid (n=1) cancer to the humerus. There were 34 males and 26 females with a mean age of 62.9±12.2. A total of 36 patients underwent EPR, while 24 patients underwent ORIF. Mean follow-up was 19.8±23.2 months. At latest follow-up, 15 patients are alive with disease and 45 patients are deceased. There was no difference in overall complication rate between endoprosthesis or plate/nail stabilization [4/36 (11%) versus 2/24 (8%); P=0.725]. Mean tumor volume was 21.9±14.8 cm3. Higher tumor volume was associated with lower MSTS scores, (r=−0.448; P=0.007).
Proximal humerus
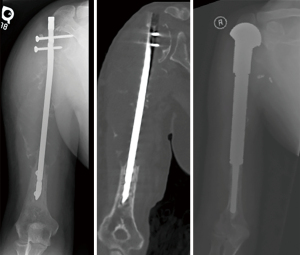
MSTS scores were comparable between ORIF and EPR [23.5/30 (78%) versus 24.0/30 (80%); P=0.834]. Overall complication rate was 5%. One patient presented to our institution after sustaining structural failure due to severe bone loss following intramedullary nailing of renal cell carcinoma. An attempt was made at limb salvage with a proximal humeral replacement, but the patient sustained deep infection which ultimately required amputation after multiple surgical attempts at limb salvage with debridement and antibiotic spacer placement (Figure 7).
Humeral shaft
MSTS scores were comparable between ORIF and EPR [25.6/30 (85%) versus 25.8/30 (86%); P=0.884]. Overall complication rate was 10.3%. Three complications occurred at the humeral shaft (2 mechanical failures in the plate/nail stabilization group, and 1 aseptic loosening in the endoprosthesis group). These complications occurred at a mean of 32 months. All were treated with revision to an intercalary endoprosthesis and have a mean MSTS score of 77% at latest follow-up. Patients with destruction of one or more cortices who were treated with plate/nail stabilization were at increased risk for mechanical failure [2/6 (33%) versus 0/18 (0%), P=0.015].
Distal humerus
MSTS scores were comparable between ORIF and EPR [23.6/30 (80%) versus 24.5/30 (82%); P=0.809]. Overall complication rate was 20%. Both patients had segmental ulnohumeral arthroplasty. Due to the anatomic location and prosthetic design, complications of aseptic loosening (n=1) and wound dehiscence (n=1) are representative of results at our institution in the primary tumor population.
Discussion
The results of this retrospective study demonstrate that cement-augmented ORIF and EPR play important roles in the management of metastatic disease to the upper extremity. Lesion location, size and remaining bone stock may guide treatment. Our data demonstrates comparable outcomes between both methods of reconstruction. We recommend consideration of an EPR in lesions at the humeral shaft associated with significant cortical destruction and in patients with certain solitary lesions, where resection may improve survival (11,12).
Previous studies have demonstrated successful outcomes with ORIF of metastatic lesions of the upper extremity (13,14). In a retrospective study of 65 patients with metastatic lesions to the humerus, Schwabe et al. (14) found ORIF at the humeral shaft to provide long-term stability and a low (7.6%) rate of complications. Bickels et al. (13) found functional outcomes to be superior when lesions were located at the humeral shaft versus at the proximal or distal humerus. We found similarly increased functional scores in patients with lesions at the humeral shaft, though these differences were not significant. Utilization of polymethylmethacrylate (PMMA) cement is important in the management of metastatic lesions following curettage. PMMA improves strength of fixation and can improve screw fixation in pathologic bone (15,16). Additionally, exothermic polymerization in PMMA minimizes blood loss and contributes to improved tumor cell destruction (17). Although chemotherapy, radiation, and bisphosphonates have contributed greatly to improved patient survival and palliation, they may attenuate the rate of biologic incorporation (6). Thus, cemented fixation was performed in all patients in this series.
Patient prognosis and radiosensitivity of lesions may play a substantial role in determining treatment choice (18). With the hematological seeding of distant tumor cells, followed by the rapid growth, invasion and destruction of surrounding bone, the biology of metastatic disease may call for a more aggressive resection and reconstruction of the bone (19-21). These considerations should be balanced with the prognosis of the individual. In addition to allowing early weightbearing, aggressive management of an isolated metastasis has shown to improve patient survival and palliation (12,22-24). In a review of 672 patients from the Scandinavian Sarcoma Group (SSG) metastasis registry, Ratasvuori et al. (12) found en bloc resection of isolated skeletal metastases of kidney, breast, lung, and prostate cancer to be associated with a significantly significant 20 month increase in survival.
While previous studies have cited improved outcomes and lower rates of re-operation with EPR at the femur (19,20,25), there is limited literature with such comparisons in metastases to the humerus. We found a low rate of complications in patients treated surgically at the humerus, limiting the statistical comparison of surgical techniques. Contrary to a systematic review by Janssen et al. (21) which found a 14–16% complication rate in patients treated with an intercalary endoprosthesis, we had one complication (5.8%) at latest follow-up utilizing a modular endoprosthetic design (10). Endoprosthetic reconstruction may inherently allow for more aggressive tumor removal and improved pain with immediate weightbearing, though this treatment decision must be balanced carefully with the risks of a potentially more extensive surgery.
There are several limitations inherent to this study’s retrospective design. We recognize that the diagnosis, medical management and prognosis of these patients over the span of our data collection has changed over time. Due to the nature of metastatic disease, long-term follow-up is limited in this patient population, as 75% of patients in this series are currently deceased. Similarly, as a tertiary referral center within our state for oncology patients, some patients were unable to be clinically evaluated in the office and followed only via the telephone and radiographs, which were sent to our institution. This, along with patients who were deceased prior to three months follow-up, led to 15 patients having MSTS functional outcome scores unavailable for analysis. The authors of this study recognize that the use of endoprosthetic reconstruction at the humeral shaft is higher than previously reported studies, though are associated with good to excellent outcomes following statistical analysis.
In conclusion, when pathologic pattern permits, cemented-augmented fixation allows for stabilization of pathologic bone, while minimizing risk of soft-tissue detachment, with mean MSTS scores of 83%. In patients with segmental defects or articular surface involvement, cemented EPR provides similar outcomes with mean MSTS scores of 83%. Patients with destruction of one or more cortices at the humeral shaft are at increased risk for mechanical failure with plate or nail stabilization. Increased tumor volume was associated with lower MSTS scores. Further studies are warranted on the association between tumor volume and method of surgical treatment in patients with metastatic disease to the humerus.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rui Yang) for the series “Bone Metastasis” published in Annals of Joint. The article has undergone external peer review.
Data Sharing Statement: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-114/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-114/coif). The series “Bone Metastasis” was commissioned by the editorial office without any funding or sponsorship. JB is a consultant and invited speaker for Merete. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The records of 229 consecutive patients treated surgically for metastatic disease from 2005–2018 at our musculoskeletal oncology center were retrospectively reviewed following institutional review board approval (Number: Pro20140000855). Inform consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Damron TA, Morgan H, Prakash D, et al. Critical evaluation of Mirels' rating system for impending pathologic fractures. Clin Orthop Relat Res 2003;S201-7. [Crossref] [PubMed]
- Manoso MW, Healey JH. Metastatic cancer to the bone. Principles and practice of oncology. 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2005:2368-80.
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004;350:1655-64. [Crossref] [PubMed]
- Houston SJ, Rubens RD. The systemic treatment of bone metastases. Clin Orthop Relat Res 1995;95-104. [PubMed]
- Janjan NA. Radiation for bone metastases: conventional techniques and the role of systemic radiopharmaceuticals. Cancer 1997;80:1628-45. [Crossref] [PubMed]
- Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 1989;256-64. [Crossref] [PubMed]
- Quinn RH, Randall RL, Benevenia J, et al. Contemporary management of metastatic bone disease: tips and tools of the trade for general practitioners. J Bone Joint Surg Am 2013;95:1887-95. [Crossref] [PubMed]
- Thurman SA, Ramakrishna NR, DeWeese TL. Radiation therapy for the treatment of locally advanced and metastatic prostate cancer. Hematol Oncol Clin North Am 2001;15:423-43. [Crossref] [PubMed]
- Benevenia J, Kirchner R, Patterson F, et al. Outcomes of a Modular Intercalary Endoprosthesis as Treatment for Segmental Defects of the Femur, Tibia, and Humerus. Clin Orthop Relat Res 2016;474:539-48. [Crossref] [PubMed]
- Lin PP, Mirza AN, Lewis VO, et al. Patient survival after surgery for osseous metastases from renal cell carcinoma. J Bone Joint Surg Am 2007;89:1794-801. [Crossref] [PubMed]
- Ratasvuori M, Wedin R, Hansen BH, et al. Prognostic role of en-bloc resection and late onset of bone metastasis in patients with bone-seeking carcinomas of the kidney, breast, lung, and prostate: SSG study on 672 operated skeletal metastases. J Surg Oncol 2014;110:360-5. [Crossref] [PubMed]
- Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am 2009;91:1503-16. [Crossref] [PubMed]
- Schwabe P, Ruppert M, Tsitsilonis S, et al. Surgical management and outcome of skeletal metastatic disease of the humerus. Acta Chir Orthop Traumatol Cech 2014;81:365-70. [PubMed]
- Harrington KD, Johnston JO, Turner RH, et al. The use of methylmethacrylate as an adjunct in the internal fixation of malignant neoplastic fractures. J Bone Joint Surg Am 1972;54:1665-76. [Crossref] [PubMed]
- Weiner M, Damron TA, Patterson FR, et al. Biomechanical study of pins in cementing of contained proximal tibia defect. Clin Orthop Relat Res 2004;232-7. [Crossref] [PubMed]
- Toy PC, France J, Randall RL, et al. Reconstruction of noncontained distal femoral defects with polymethylmethacrylate and crossed-screw augmentation: a biomechanical study. J Bone Joint Surg Am 2006;88:171-8. [Crossref] [PubMed]
- Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br 2001;83:471-81. [Crossref] [PubMed]
- Janssen SJ, Kortlever JT, Ready JE, et al. Complications After Surgical Management of Proximal Femoral Metastasis: A Retrospective Study of 417 Patients. J Am Acad Orthop Surg 2016;24:483-94. [Crossref] [PubMed]
- Wedin R, Bauer HC. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J Bone Joint Surg Br 2005;87:1653-7. [Crossref] [PubMed]
- Janssen SJ, Teunis T, Hornicek FJ, et al. Outcome of operative treatment of metastatic fractures of the humerus: a systematic review of twenty three clinical studies. Int Orthop 2015;39:735-46. [Crossref] [PubMed]
- Jung ST, Ghert MA, Harrelson JM, et al. Treatment of osseous metastases in patients with renal cell carcinoma. Clin Orthop Relat Res 2003;223-31. [Crossref] [PubMed]
- Henrichs MP, Krebs J, Gosheger G, et al. Modular tumor endoprostheses in surgical palliation of long-bone metastases: a reduction in tumor burden and a durable reconstruction. World J Surg Oncol 2014;12:330. [Crossref] [PubMed]
- Utzschneider S, Weber P, Fottner A, et al. Prognosis-adapted surgical management of bone metastases. Orthopade 2009;38:308-310-12, 314-5. [Crossref] [PubMed]
- Di Martino A, Martinelli N, Loppini M, et al. Is endoprosthesis safer than internal fixation for metastatic disease of the proximal femur? A systematic review. Injury 2017;48:S48-S54. [Crossref] [PubMed]
Cite this article as: Ippolito JA, Thomson JE, Lelkes V, Amer K, Patterson FR, Benevenia J, Beebe KS. Cemented-augmented fixation of metastatic humeral lesions without segmental bone loss results in reliable outcomes. Ann Joint 2022;7:13.


