
Surgery for proximal femur metastases: endoprosthesis reconstruction or intramedullary nailing?
Introduction
Recently, advanced oncological treatment has increased the life expectancy of patients with cancer and the incidence of skeletal metastases (1). Up to 70% of patients with malignant tumors sustain a fracture, and 65% of such fractures requiring surgery occur in the femur (2,3). The proximal femur is a major load-bearing region that is constantly at stress during load transmission, and its structural integrity is essential for mobility.
Metastases to the proximal femur with an impending or pathological fracture result in severe pain and restricted movements. Proximal femur metastases must be addressed surgically to relive pain and allow early mobilization, thereby improving the patient’s quality of life. There are numerous options for surgical stabilization of such lesions; an ideal implant should be biomechanically competent to withstand the substantial load around the region, have increased longevity, and possess minimal complication rates. The characteristics of the lesion and the patient’s general condition have to be considered before deciding on the method of reconstruction for proximal femur metastases. Endoprosthesis (EP) reconstruction and intramedullary nailing (IMN) are the two commonly used reconstruction methods, and each has its own reported advantages and disadvantages (4,5).
The optimal choice of implant for surgical stabilization of proximal femur metastatic lesions is controversial, and there is an ongoing debate on the recommendation of the type of implant (6). In this regard, this study aimed to compare the outcome between EP reconstruction and IMN for proximal femur metastases in a large institutional series.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/aoj-20-96).
Methods
Study design, setting, and participants
A retrospective review was performed on the prospectively collected institutional database of 117 patients who underwent surgery for proximal femur metastases between January 2012 and December 2017. For the analyses, patients with the following conditions were excluded from the study: (I) metastases of the femoral head or neck without trochanteric extension that is not an indication for intramedullary fixation (n=18); (II) previous surgery to the ipsilateral femur (n=8); (III) surgeries other than IMN or EP reconstruction (n=7); and (IV) concomitant metastases in the contralateral or ipsilateral femur warranting surgery (n=6). Of the remaining 78 patients, 8 patients with <3 months of follow-up postoperatively were excluded, leaving 70 patients for the analyses.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Seoul National University Hospital (No. 1812095996) and individual consent for this retrospective analysis was waived.
The indications for surgery for proximal femur metastatic lesions were impending fracture as per Mirel’s criteria (7), pathological fracture, and debilitating pain. All surgeries were performed by two senior surgeons (HSK and IH). Of the 70 patients, 37 patients were reconstructed with IMN, which was primarily applied in the early part of our series from 2012 to 2015. Since 2015, EP reconstruction was more common and performed in 33 patients. Surgical options of IMN or EP reconstruction were based on multiple factors, such as patient preference, extent of cortical destruction, radiological pattern of the lesion (lytic vs. sclerotic), presence or absence of fracture displacement, primary diagnosis, response to adjuvant therapy, expected survival time, and surgeon’s preference.
Description of the treatment
In the IMN group, reconstruction was performed using either Zimmer natural nail (Zimmer Inc., Warsaw, IN, USA) (n=15) or proximal femoral anti-rotation nail (Synthes Inc., West Chester, PA, USA) (n=22). The femoral canal was not reamed, and the nail with the largest possible diameter was inserted. The cavity with the metastatic lesion was thoroughly curetted, and bone cement was used as structural support to fill the void following curettage, after the nail was inserted. Postoperatively, if the reconstruction was stable, weight bearing as tolerated was initiated in the first or second postoperative day using a walker. If IMN was performed in patients with pathological fracture or the construct was not stable, weight bearing was delayed for 3 weeks. In the EP group, cemented proximal femoral EP (Modular Universal Tumor and Revision System; Implantcast GmbH, Buxtehude, Germany) was used in 23 patients, and conventional cemented hip hemiarthroplasty was performed in 10 patients (IC straight stem; Implantcast GmbH, Buxtehude, Germany, in six patients and VerSys® cemented CT; Zimmer Inc., Warsaw, IN, USA, in four patients). Bipolar head components were used in all patients, and no acetabular resurfacing was performed in this series. In patients undergoing EP reconstruction, the tendons of the gluteus medius, iliopsoas, and vastus lateralis muscles were reattached using the Trevira tube (Implantcast Inc., Buxtehude, Germany), and the hip capsule was closed in all patients. These patients were allowed weight bearing as tolerated during the first or second postoperative day. Patients initially used a walker and progressed to using crutches and subsequently without support, as tolerated. Patients did not use brace postoperatively.
Data sources, variables, and outcome measures
Demographic details on presentation; characteristics of the proximal femur metastatic lesion on plain radiographs, CT images, and MRI; details of primary cancer and treatment received; details of the surgical procedure; and postoperative details, such as complications and outcomes of the procedure and functional status of the patient, were retrieved from the database.
Complications at the operated site in the postoperative period that needed intervention, such as local recurrence (LR), infection, peri-implant fracture, and implant failure, and systemic complications following surgery, such as pleural effusion, pneumonia, deep vein thrombosis, and pulmonary thromboembolism, were noted. Implant survival was defined as the percentage of patients with a particular implant, which was retained without removal or revision of any or all its components, until death or last follow-up. During the postoperative period, LR was defined as radiological identification of tumor recurrence at the operated tumor bed. LR-free survival was defined as the period between the date of surgery and date of LR or date of last imaging. Functional outcomes were assessed using the Musculoskeletal Tumor Society (MSTS) system, which includes parameters of pain, function, emotional acceptance, need for external support, walking ability, and gait (8).
The following factors were compared between the IMN and EP groups: incidence of postoperative complications, overall survival, LR-free survival, implant survival, MSTS scores at 6 months and 1 year following surgery, maximum ambulatory ability of the patient following surgery, and time taken to ambulate independently without support.
Demographic details
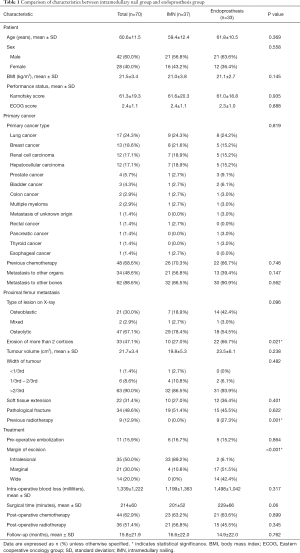
There were 42 men and 28 women with a mean age of 61 years (range, 33–86 years) (Table 1). The most common primary cancers were lung cancer (n=17), breast cancer (n=13), renal cancer (n=12), and hepatocellular carcinoma (n=12). Nine patients (12.9%) had received radiotherapy preoperatively at the operative site. Sixty-two patients (88.6%) had metastasis to the bones apart from the proximal femur, and 34 (48.6%) had metastasis to other organs. Forty-seven (67.1%) patients had osteolytic lesion of the proximal femur, and the width of the tumor was more than two-thirds of the diameter of the bone in 90% (n=63) of patients. Thirty-four patients (48.6%) presented with pathological fracture of the proximal femur. Soft tissue extension of the tumor was observed in 31.4% (n=22) of patients. With respect to the preoperative functional status, 66% (n=46) of patients had an Eastern Cooperative Oncology Group scores of 2 or 3, and the mean Karnofsky score was 61.3±19.3 (9). Resection margins were intralesional in 35 patients (50%), marginal in 21 (30%), and wide in the remaining 14 (20%). Preoperative embolization was performed in 11 patients (16%). Patients were followed for a mean duration of 15.4 months (range, 3–109 months). Between the IMN and EP groups, there was no significant difference in preoperative patient characteristics, such as age, sex, body mass index, and performance status. With respect to the preoperative characteristics of the proximal femur metastatic lesion, a significantly higher number of patients in the EP group had erosion of more than two cortices (P=0.021) and received radiotherapy (P=0.001) compared to those in the IMN group.
Full table
Statistical analysis
Continuous variables were presented as mean with standard deviation, and analysis of variance was used for statistical analysis. Categorical variables were presented as frequencies with percentage, and chi-square test was used. A P value <0.05 indicated statistical significance. Difference in postoperative complications between the IMN and EP groups were determined using Fisher’s exact test. Kaplan-Meier survival curves were used to determine the difference in LR-free survival, implant survival, and overall survival of patients. Log-rank analysis was used to determine the significance of difference between the survival curves. Statistical analyses were performed using the SPSS software (version 23.0; IBM Co., Armonk, NY).
Results
Complications
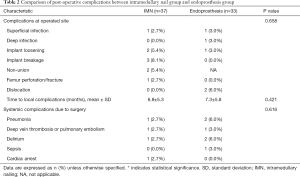
Apart from LR, 24.3% (9 of 37) of patients in the IMN group and 15.1% (5 of 33) of patients in the EP group developed complications at the operative site (P=0.658) (Table 2). Implant breakage was the most common complication in the IMN group (n=3) and dislocation of the bipolar head was the most common complication in the EP group (n=2). The time to complication since surgery was comparable between the two groups (6.8±5.3 vs. 7.3±5.8 months, P=0.421).
Full table
Systemic complications attributable to surgery was noted in 10.9% (4 of 37) and 18.2% (6 of 33) of patient in the IMN and EP groups, respectively (P=0.616).
Local recurrence
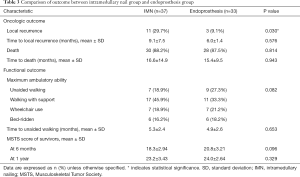
Among the 70 patients in the study, 14 patients (20%) developed LR after a mean time of 8.7 months (range, 1–23 months). The IMN group showed significantly higher LR rate than the EP group (29.7% vs. 9.1%, P=0.030) (Table 3). However, the mean time to LR was not statistically different between the two groups (9.1±7.5 months for the IMN group vs. 6.0±1.4 months for the EP group, P=0.576). On Kaplan-Meier analysis, the IMN group had significantly lower LR-free survival than the EP group (P=0.002) (Figure 1). Of 11 patients with LR in the IMN group, four patients were treated surgically, three patients underwent local radiation, two patients were treated with chemotherapy, and the remaining two patients did not receive treatment. Among the three patients with LR in the EP group, one patient underwent surgery, one patient received radiotherapy, and the other patient underwent chemotherapy.
Full table

Functional outcome
There was no statistically significant difference in the maximum ambulatory ability between the IMN and EP groups (P=0.082). A majority of the patients in both groups (45.9% in the IMN group and 33.3% in the EP group) were ambulatory with support at the final follow-up or death. Among the 19% (n=7) of patients in the IMN group and 27% (n=9) of patients in the EP group who could ambulate without any support, the time taken for independent ambulation from surgery was similar (5.3±2.4 months in the IMN group vs. 4.9±2.6 months in the EP group, P= 0.653). The MSTS scores of the survivors at 1-year follow-up were also similar between the two groups (23.2±3.43 in the IMN group vs. 24.0±2.64 in the EP group, P= 0.329).
Implant and patient survival
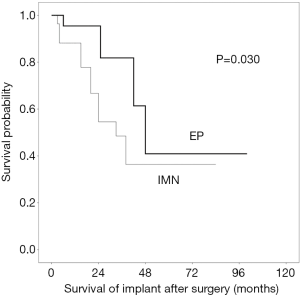
At the final follow-up or death, 10 patients (27%) who underwent IMN required implant revision (5 exchange nailing and 3 EP conversion, at a mean interval of 11.7±14.7 months (range, 1–41) from the primary surgery. In the EP group, five patients (15%) underwent revision of the implant at a mean interval of 14.2±16.9 months (range, 1–51) from the primary surgery. On Kaplan-Meier analysis, the implant survival at 2 years postoperatively was significantly better in the EP group (83%) compared to that in the IMN group (54%) (log rank, P=0.030) (Figure 2). The most common cause of revision was LR (n=4, 40%) in the IMN group and dislocation (n=2, 40%) in the EP group.
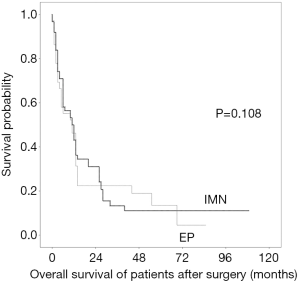
Of the 70 patients in the study, 58 (87.9%) had died until the last follow-up. The actuarial survival rates calculated at 6 months, 1 year, and 2 years were 64.5%±4.4%, 49.6%±4.5%, and 30.3±3.8%, respectively. There was no statistically significant difference in overall survival at 3 years postoperatively between the two groups on Kaplan-Meier analysis (17.8% for the IMN group vs. 14.5% for the EP group, P=0.108) (Figure 3).
Discussion
The proximal femur is the most common site of skeletal metastases in the appendicular skeleton. Pain relief and early mobilization are the goals of surgery for such lesions. The choice of implant for surgery depends upon the type and extent of the lesion and general condition of the patient. IMN and proximal femur EP reconstruction are the two commonly used methods; however, there are no guided protocols determining the use of one implant over the other (10). We performed a retrospective review on patients with proximal femur metastases, undergoing surgery with IMN and EP, to compare the perioperative characteristics, clinical outcome, and implant survival between the two groups. The functional outcome and postoperative complication rate were similar between the two groups, but the LR-free survival and implant survival were significantly better in the EP group.
Perioperative characteristics
Approximately 67% of patients with EP in our study had destruction of more than 2 cortices of the femur due to metastasis, compared to 27% of patients in the IMN group (P=0.021). Extensive bone loss or destruction warrants the need for EP reconstruction over IMN. Radiotherapy to the site of metastasis can cause loss of soft tissue vascularity and elasticity and render the environment immunosuppressed and malnourished. Poor soft tissue condition is a significant risk factor for failure of limb salvage surgery (11). In our study, all patients who had received radiotherapy preoperatively to the site of metastases were included in the EP group (P=0.001). Improved radiation delivery techniques, new radiation modalities, and low toxicity radiosensitizing agents have reduced the local tissue damage, and megaprostheses have been used safely for orthopedic reconstructions (12). In our study, blood loss in the EP group was 1,498±1,042 mL and was higher but not significant (P=0.317) compared to that in the IMN group, with 1,199±1,363 mL. Some studies have reported less blood loss in the IMN group compared to that in the EP group, as tumor tissue was not removed and only in situ nail fixation was performed in the IMN group, contrary to our practice of thorough curettage of the metastatic lesion with a curative intent (13). Although there was no statistically significant difference (P=0.06), the surgical time was longer in the EP group in our study. EP reconstruction requires more soft tissue dissection and reconstruction (gluteus medius and maximus, hip joint capsule, vastus lateralis) compared to IMN, prolonging the surgical time.
Outcome
Complications
In our study, the complications at the operative site and systemic complications were similar between the two groups. Yu et al. reported increased complication rate in the IMN group compared to that in the EP group in their retrospective review of 88 patients who underwent surgery for proximal femur metastasis (13). Some studies attribute the complications of IMN to reaming of the intramedullary canal causing local and systemic dissemination of tumor tissue, leading to pulmonary embolism, and mechanical inadequacies of the nail, which can lead to implant failure. However, a recent systematic review reported increased complication rate of EP reconstruction compare to that of IMN, such as dislocations and infections, which can be attributed to extensive soft tissue dissection during EP reconstruction (14).
Oncological outcome
LR-free survival was significantly lower in the IMN group (P=0.002), in line with the literature (13). The use of EP allows en bloc resection of the tumor-bearing bone, which permits better clearance of the tumor tissue, resulting in the reduction of LR and need for postoperative radiotherapy. En bloc resection of solitary bone metastasis may improve overall survival (15).
Functional outcome
We found no difference in functional outcome between the two groups as MSTS scores at 6 months, 1 year, and final follow-up were similar. Yu et al. reported better early functional outcome (MSTS score at 6 weeks) in patients with IMN as it did not involve resection of the muscles around the hip, whereas Guzik et al. reported better functional outcome in the EP group, as 31% (23/75) of these patients were ambulatory without crutches by 3 months, which was not possible in any of the 26 patients with IMN (13,16). IMN may allow faster recovery, whereas EP reconstruction allows early independent limb function and mobilization, both of which are important to patients with bone metastasis due to their limited survival time (13).
Implant survival
In our study, implant survival was significantly better in the EP group compared to that in the IMN group (P=0.030). The most common cause for revision of implant in the IMN group was LR, which was less in patients with EP. Apart from LR, implant failure was also more frequently observed in the IMN group. The IMN is a load-sharing device that is supposed to gradually transfer the load to the bone as it heals. Incomplete removal of tumor tissue, LR, delayed healing of the bone due to adjuvant radiotherapy, or any other factor that contributes to poor bone quality leads to IMN bearing the entire load and implant failure (17). However, EPs are load-bearing devices that allow immediate weight bearing and reduced mechanical failure and longer implant survival (4,18). As expected, patients with a longer postoperative survival time had a higher rate of revision surgery. Moreover, 48% of patients who survived >1 year after surgery for bone metastasis necessitated revision surgery compared to 29% and 36% of patients who survived for 3 and 6 months, respectively. These findings highlight the necessity of accurate prediction of postoperative survival time.
Patient survival
Janssen et al. reviewed 417 patients with proximal femur metastasis, and 58% of these patients were alive by the end of 3 months, and only 25% were alive at 1 year postoperatively (19). Harvey et al. reported survival of 51%, 29%, and 11% at 1, 2, and 5 years respectively, which was similar to our study (17). Various survival outcomes have been reported in the literature, but there is no level I evidence showing improved overall survival of patients with one implant over the other, and eventually both implants have outlived the patients, considering the relatively short lifespan of the patients (18,20).
Limitations
The study had a number of limitations. First, the patients were not randomized, and the study design was retrospective. However, a large number of consecutive patients prospectively collected from a database were included in both groups. Second, patients with different cancers were included, and the details of treatment of the primary cancer were not considered in the analysis. Third, the implant selection was subjective, solely based on surgeon’s experience, which can lead to treatment bias. However, in comparing the characteristics of the lesion between the IMN and EP groups, in patients with destruction of more than two cortices and pathological fracture, EP was used more frequently. Hence, the surgeon’s indications for use of a particular implant were clear, thereby reducing the treatment bias. Lastly, the outcomes were only compared between the IMN and EP group, and other patient and tumor characteristics might be confounding factors for the outcome. A randomized prospective analysis with a long-term follow-up is essential to provide greater degree of evidence for the use of a specific type of implant.
Conclusions
The LR-free survival and implant survival are better with prosthetic reconstruction compared with use of intramedullary devices for proximal femoral metastatic lesions. As the complication rates and functional outcome of patients with both implants are comparable, prosthetic reconstruction can be safely used to provide better durability even in patients with a shorter life span to obtain the best quality of life.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rui Yang) for the series “Bone Metastasis” published in Annals of Joint. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/aoj-20-96
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aoj-20-96
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-96). The series “Bone Metastasis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Seoul National University Hospital (No. 1812095996) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bryson DJ, Wicks L, Ashford RU. The investigation and management of suspected malignant pathological fractures: a review for the general orthopaedic surgeon. Injury 2015;46:1891-9. [Crossref] [PubMed]
- Choy WS, Kim KJ, Lee SK, et al. Surgical treatment of pathological fractures occurring at the proximal femur. Yonsei Med J 2015;56:460-5. [Crossref] [PubMed]
- Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am 2000;31:515-28. vii. [Crossref] [PubMed]
- Selek H, Basarir K, Yildiz Y, et al. Cemented endoprosthetic replacement for metastatic bone disease in the proximal femur. J Arthroplasty 2008;23:112-7. [Crossref] [PubMed]
- Samsani SR, Panikkar V, Venu KM, et al. Breast cancer bone metastasis in femur: surgical considerations and reconstruction with Long Gamma Nail. Eur J Surg Oncol 2004;30:993-7. [Crossref] [PubMed]
- Issack PS, Barker J, Baker M, et al. Surgical management of metastatic disease of the proximal part of the femur. J Bone Joint Surg Am 2014;96:2091-8. [Crossref] [PubMed]
- Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 1989;256-64. [Crossref] [PubMed]
- Enneking WF, Dunham W, Gebhardt MC, et al. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993;241-6. [Crossref] [PubMed]
- Verger E, Salamero M, Conill C. Can Karnofsky performance status be transformed to the Eastern Cooperative Oncology Group scoring scale and vice versa? Eur J Cancer 1992;28A:1328-30. [Crossref] [PubMed]
- Feng H, Wang J, Xu J, et al. The surgical management and treatment of metastatic lesions in the proximal femur: A mini review. Medicine (Baltimore) 2016;95:e3892 [Crossref] [PubMed]
- Hardes J, Gebert C, Schwappach A, et al. Characteristics and outcome of infections associated with tumor endoprostheses. Arch Orthop Trauma Surg 2006;126:289-96. [Crossref] [PubMed]
- Mavrogenis AF, Pala E, Romantini M, et al. Side Effects of Radiation in Musculoskeletal Oncology: Clinical Evaluation of Radiation-Induced Fractures. Int J Immunopathol Pharmacol 2011;24:29-37. [Crossref] [PubMed]
- Yu Z, Xiong Y, Shi R, et al. Surgical management of metastatic lesions of the proximal femur with pathological fractures using intramedullary nailing or endoprosthetic replacement. Mol Clin Oncol 2018;8:107-14. [PubMed]
- Di Martino A, Martinelli N, Loppini M, et al. Is endoprosthesis safer than internal fixation for metastatic disease of the proximal femur? A systematic review. Injury 2017;48:S48-S54. [Crossref] [PubMed]
- Ratasvuori M, Wedin R, Hansen BH, et al. Prognostic role of en-bloc resection and late onset of bone metastasis in patients with bone-seeking carcinomas of the kidney, breast, lung, and prostate: SSG study on 672 operated skeletal metastases. J Surg Oncol 2014;110:360-5. [Crossref] [PubMed]
- Guzik G. Oncological and functional results after surgical treatment of bone metastases at the proximal femur. BMC Surg 2018;18:5. [Crossref] [PubMed]
- Harvey N, Ahlmann ER, Allison DC, et al. Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin Orthop Relat Res 2012;470:684-91. [Crossref] [PubMed]
- Wedin R, Bauer HC. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J Bone Joint Surg Br 2005;87:1653-7. [Crossref] [PubMed]
- Janssen SJ, Kortlever JT, Ready JE, et al. Complications After Surgical Management of Proximal Femoral Metastasis: A Retrospective Study of 417 Patients. J Am Acad Orthop Surg 2016;24:483-94. [Crossref] [PubMed]
- Sarahrudi K, Hora K, Heinz T, et al. Treatment results of pathological fractures of the long bones: a retrospective analysis of 88 patients. International Orthopaedics. Int Orthop 2006;30:519-24. [Crossref] [PubMed]
Cite this article as: Hindiskere S, Kim HS, Kim Y, Han I. Surgery for proximal femur metastases: endoprosthesis reconstruction or intramedullary nailing? Ann Joint 2021;6:27.


