The direct anterior approach to the hip for total hip arthroplasty: a blind guide (with traction table)
Introduction
The direct anterior approach (DAA) to the hip is gaining international popularity due to its well-known and documented advantages including muscle sparing nature of the approach, early kinematic recovery, and low dislocation rate (1). Furthermore, evidence shows that the approach provides good functional outcome (2) as demonstrated by improvements in cadence, step and stride time at two years after surgery (2). It has been concluded that the inter nervous and muscle-sparing nature of the DAA enables the surgeon to aid in preservation of muscle function resulting in significant differences in gait when compared to other approaches, not only within the first 12 weeks post-surgery (3) but also at two years (2). The DAA was found to offer significant early advantages in function when compared to the direct lateral approach (DLA), with no differences in quality of life or pain in a randomised control trial comparing gait analysis and patient reported outcome measures (PROMS) for these two approaches (4).
This approach was first described by Carl Heuter in 1881, but popularised by Smith-Peterson in 1911 (5). It employs the intermuscular and internervous planes between Sartorius (Femoral nerve) and TFL (Superior Gluteal Nerve) superficially and between Gluteus Medius (Superior Gluteal Nerve) and Rectus (Femoral nerve) in the deep layer (5,6). The senior author utilises the DAA as his main surgical approach to the hip based on the modified Heuter approach (7) where its indication is based on surgeon experience and preference. As for patient factors, patient selection is similar to those for other approaches to the hip with special attention paid to those patients who have a large panniculus where their skin overlies the surgical field of DAA which will experience different stresses, moisture levels, and has a higher incidence of fungal colonization, especially in the obese patients (8). The presence of hardware from previous fixation or osteotomy can affect the decision of choosing the preferred approach. This approach is used for primary Total Hip arthroplasty (THA) and revision cases without compromising nerve supply to muscles and, when necessary, an anterior femoral osteotomy can be used in cases when a well-fixed or osseointegrated implant is encountered and is difficult to remove (9). This approach can also be safely used for the treatment of periprosthetic femoral fractures (PFFs) (10) and two-stage revision secondary to periprosthetic infection (PPI) with good eradication rate (11). Our institution has adopted an enhanced recovery program and have strived for outpatient surgical strategies in these procedures utilising the DAA. At our institution, on select, well-informed patients, simultaneous bilateral THAs through the DAA with low perioperative complications can be consistently performed, however it was seen to have more intraoperative blood loss (averaged 632 mL per one setting) requiring 33% of the patients to receive blood transfusions from their cell saver (12). A study from our institution found that simultaneous bilateral THAs is more cost effective than staged counterpart with no added risk (13,14). Furthermore, it was found that the DAA to the hip is more cost effective than the DLA and the posterior approach in a cost analysis study performed at our institution (15).
Our aim is to present a step-by-step description of our DAA to aid surgeons who are interested in learning this approach using a special traction table.
Set up
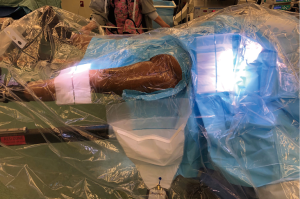
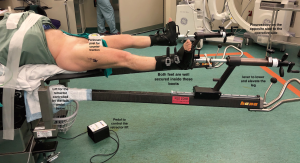
The patient is positioned on a specialised traction table (1,5,15) (Hana fracture table, Mizuho, OSI) (Figure 1). This approach can also be done without a traction table (5), but it will only be described using a traction table in this guide. The position of the patient is supine where the pelvic position is more reliable, leading to more consistent acetabular component orientation (40/20 + −10 of inclination/anteversion) as was found by Grammatopoulos et al. comparing supine to the lateral decubitus position (16). Both feet are wrapped with a special adhesive bandage and placed into the traction boots for added grip, allowing intraoperative manoeuvring and traction of the operative leg at the end of the table. The perineum post is secure in the middle to provide counter traction during the procedure. The legs are positioned spread apart in more abduction to allow easier draping with less possibility of contamination (Figure 2).
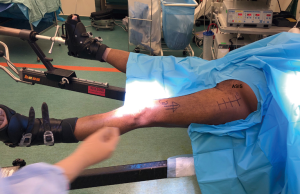
The skin is marked prior to the application of a sterile transparent adhesive cover. A circle is drawn around the anterior superior iliac spine (ASIS), followed by a line 2-4 cm lateral to the circle directed laterally towards the lateral border of the ipsilateral patella approximately 10–14 centimetres in length (1) (Figure 2). In cases involving PFFs and revision THAs, this approach can be safely extended distally using a “lazy S” shape incision combined with proximal extension of the approach to the attachment of the TFL (10). A sterile U drape is applied first to isolate the operative area (Figure 2) followed by a special transparent sterile drape to cover the entire body (figure 3) stopping at the level of the feet to give freedom to an un-scrubbed assistant for manoeuvring the leg. When there is no assistant, the foot should be covered with a sterile drape to allow the surgeon to manoeuvre the operative extremity.
Surgical procedure
The skin Incision is made using a scalpel, followed by diathermy to clear the fat within the subcutaneous layer down to fascia. Sterile gauze is used to sweep the fat medially to expose the fascia over the TFL. Only sometimes the lateral femoral cutaneous nerve of the thigh is identified and when this happen it is subsequently protected by mobilizing it medially (1). In the event that two layers of fascia are encountered, one should confirm that the approach has not drifted too medially. The fascia is then split at this point inline with skin incision to expose the superficial intermuscular interval between tensor fascia lata (TFL) and Sartorius. Great care is needed here to ensure the Sartorius isn’t confused with the TFL, as this results in attempting to use the interval between the neurovascular bundle and the Sartorius. The orientation of the muscle fibers (distal and medial as compared to distal and lateral typical of the TFL) as well as the point of insertion is helpful. It is important to avoid splitting the Sartorius or the TFL to diminish muscle damage. Once through the superficial layer, blunt dissection is used to sweep the muscles, exposing the correct interval within the deep layer (Rectus Femoris and Gluteus Medius) until a soft spot on the superior - lateral aspect of the femoral neck is identified. A blunt-end long Cobra retractor is used to replace the finger over the superior - lateral aspect of the femoral neck. A Charnley retractor is then used with the deep blade attachments in order to retract the TFL laterally and the rectus medially. At this point, diathermy is used for deeper dissection to the capsule until the branches of the ascending lateral circumflex femoral artery become more visible. Forceps are used to cauterise these branches. This step is to be done thoroughly and with care to minimize blood loss. A Rongeur is used to remove the fat superior to the capsule. A second long tip cobra retractor is used to bluntly pass medial to the medial edge of the femoral neck. At this point these two Cobra retractors are outside the capsule over the lateral and medial femoral neck edges. S shaped capsulotomy is made, starting on the ilium just above the acetabulum then heading distally and laterally to the intertrochanteric line where medial and lateral capsular flaps are created. The S shape capsulotomy is used to optimize exposure and diminish soft tissue tension. Starting the capsulotomy on the iliac wing and elevating a small portion posteriorly allows the femur to sag posteriorly slightly, opening the exposure to the acetabulum. Extending the capsulotomy along the superior aspect of the femoral neck maximizes the anterior capsular flap, to allow greater protection of the neurovascular bundle if using a Charnley retractor or other medial retractors. The capsule is lifted off the anterior aspect of the femur in a similar fashion T shaped capsulotomies.
The flaps can be secured with a number 2-ethibond stay suture that can aid in capsular closure at the end of the case. The two stay sutures are differentiated by placing a Kelly on the anterior suture and the posterior suture is left dangling without a clamp attached. This is our preferred way to deal with the capsule, however some authors routinely perform a partial or complete capsulectomy (5). In revision cases, when more exposure is necessitated, it can be helpful to perform capsulectomy, thereby improving our visualization. It is important to place medial aspect of the Charnley inside the capsule as soon as possible to protect the neurovascular bundle.
At this point the lateral cobra retractor is placed inside the capsule and the medial cobra retractor is replaced with a double-pronged Muller retractor and placed even deeper to help clear the medial side of the femoral neck by peeling off capsule medially. With the goal of adequate visualization of the proximal femur, diathermy is used to further clear the medial femoral neck distal to the intertrochanteric line as well as laterally by clearing up the saddle of the greater trochanter (GT).
To make the femoral neck osteotomy, the leg is placed in neutral position, which helps keep the GT lateral while making the femoral neck osteotomy. The level of femoral neck osteotomy is decided upon based on preoperative templating where the height from the lesser trochanter must be decided preoperatively as well as from the saddle of the femoral neck transition to the greater trochanter. Typically, the neck cut is made with primary reference to the saddle and at a constant angle. However, this can be reconfirmed as well during the operation using the first broach or even the special neck cut guide provided within the set. With the leg in traction, a sagittal reciprocating saw is used for the femoral neck cut starting at the medial anterior cortex and moving laterally only through the anterior cortex. This allows us to gauge the depth of the femoral neck and aid in avoiding transecting the deeper vascular structures. The posterior cortex is then cut, taking great care to minimize the saw excursion. As the cut carries us laterally near the GT, the saw trajectory is then changed from a medial-to-lateral path to a more vertical path, starting from the saddle and progressing to the previously made neck cut, which helps avoid injury to the GT. An osteotome is subsequently used to separate the head from the femoral neck and completing the osteotomy. External rotation of the leg at this point displays the cut surface of the femoral head and allows the introduction of a corkscrew for removal of the head. Using the corkscrew engaged in the femoral head, the head should be circumducted with concurrent traction until the soft tissue attachments are freed, allowing removal of the head from the socket.
At this point, the leg is externally rotated to allow verifying the level of the cut from the lesser trochanter. As part of our exposure, the pubio-femoral ligament is released to allow better exposure on anteromedial then posteromedial part of the proximal femur and to facilitate checking the lesser trochanter level. Sometimes this cut can be readjusted by using the saw or calcar reamer when a need to shorten the cut is required after broaching and trialling.
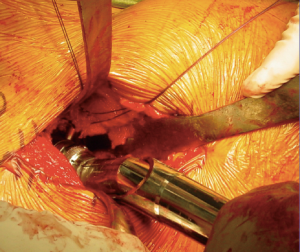
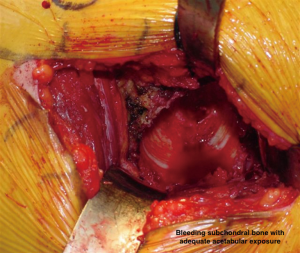
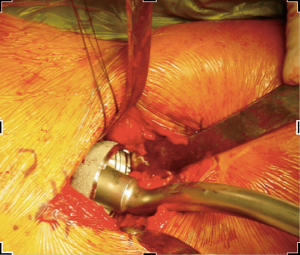
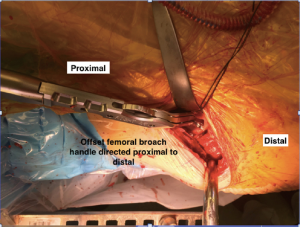
In order to gain visualization of the acetabulum, a blunt cobra is placed into the posterosuperior position of the acetabulum. The labrum is excised in a standard fashion, using a long-handled scalpel and grasper. At this point, the process of reaming the acetabulum is begun, where all reaming is done with the use of an offset handle attachment (Figures 4,5). Our first reamer is usually performed with size 44 directed medially (Figure 5), which allows us to ream to the true floor of the acetabulum. Next, the reamer size is sequentially increased maintaining the typical desired position of the cup which is an abduction angle of approximately 40–45 degrees and anteversion around 20–25 degrees. Once there is satisfaction with the acetabular reaming evidenced by adequate bleeding subchondral bone in the desired position (Figure 6), an offset acetabular component inserter is then used (Figures 4,7) to place the socket under direct fluoroscopy guidance. Using a mallet, the cementless acetabular shell is impacted into the ideal position of abduction and anteversion and subsequently verified using fluoroscopy. Screws can also be inserted to augment acetabular fixation at surgeon’s discretion (15). After the acetabular cup is satisfactorily seated, the final polyethylene liner is placed.

Next, the attention is turned to the femur. First, all retractors must be removed from the operative field and then the leg is positioned in the neutral position in order to bring the GT laterally. Using the operator’s fingers, staying on bone, the posterolateral tissue around the GT is swept down to the insertion of Gluteus Maximus, which allows a pouch to be created which facilitates the elevation of the femur using a special retractor and break down the GT bursae. A special femoral elevator hook retractor with its base attached to the table (Figures 1-8) is positioned and subsequently can be controlled using a foot pedal to elevate or lower the retractor. The leg is slowly extended in neutral rotation while femur is being brought anteriorly, the leg is typically extended approximately 45 degrees or until the proximal femur is delivered with enough access for the next step, soft tissue releases. It is important not to engage the retractor to the table until the leg is in extension to avoid fracture. Anterior tension can then be placed on the retractor using the foot pedal. A double-pronged retractor (Muller) is used in order to push the femur laterally and then followed by the placement of a second double-pronged retractor where it is inserted posteriorly to the GT and lateral to the posterior capsular leaflet. At this point this leaflet is held by the stay suture or with a grasper and using a diathermy this leaflet is separated from the short external rotators (mainly Piriformis). With the use of the diathermy the posterior capsule attachment is released by grasping and pulling it inside of the GT and the piriformis fossa to create traction and in a distal to proximal motion, combined with a medially directed path so that the posterior capsule can be adequately released (Figure 9). There will be a subtle anterior motion of the proximal femur when the appropriate release of the proximal femur has been performed, the proximal Muller should be adjusted now to be passed deep behind the GT to improve exposure.
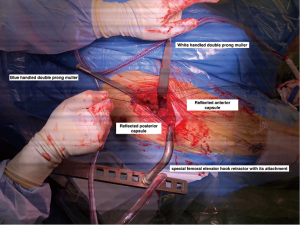
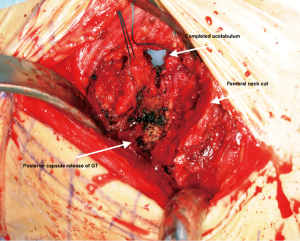
There are occasional circumstances that merit the need to release the conjoint tendon completely, more than what was previously described, in order to allow further elevation of the femur by identification of the tendon and using diathermy to release its attachment. Care should be taken not to release the obturator externus. Figure 8 shows the position of three retractors and the femoral cut after releasing the tendon. It is acceptable to have a low threshold to perform this step in order to improve exposure with no identified effect on the postoperative gait as shown in our study comparing THA through this approach with and without release (17) Although the conjoint tendon has an important role in the biomechanics of the hip, our institution found that releasing this tendon during the DAA has no impact on gait or patient reported outcomes (PROMs) within 12 weeks post-surgery (17). However, our institution performed a gait analysis study and found that by releasing these muscles it can affect the internal rotation moment (18). This is probably not clinically significant as demonstrated from PROMs following the release, as was discussed above. (17). In another retrospective cohort, a study out of our institution concluded that the release of conjoint tendon had no effect on length of stay, functional outcomes at 1 year or requirement for pain medications (19).
An aggressive starting broach (Figure 4) is used to open the canal of the proximal femur in the desired anteversion orientation utilising an offset broach handle (Figures 4,10). This is followed by sequential rasping towards a suitable trial broach size. With his/her back to the patient’s feet, the surgeon directs the broaches laterally with an internal rotation force on entry, and a gentle medial force on removal to ensure the line of broaching is appropriate. It is important to make sure that the correct stem size is used, as there is a tendency to under rasp and under size the stem due to the fear of fracture. However, with the use of intraoperative fluoroscopy it can help to maximise stem size as some evidence has shown significant earlier numbers of revisions after the THAs utilizing the DAA due to aseptic loosening of the femoral stem when compared to other approaches (20). The aseptic loosening is likely caused by undersizing the femoral component due to poor visualisation of the proximal femur and/or occult femoral fractures leading to early subsidence and aseptic loosening (20). It is highly recommend that intraoperative fluoroscopy is used in order to avoid under sizing the stem as demonstrated by Rivera et al. (21). At this juncture, the stem should be in the correct orientation otherwise it could result in fracture and failure to advance the femoral stem which is usually caused by poor hand positioning. It is important to keep the hand on the handle pressed down to the floor keeping the stem out of extension as well as forceful prevention of anteversion during broach or stem advancement. The trial neck should be used at this point. The leg should be externally rotated to allow the trial head to be inserted onto the trial neck, sometimes a hook may be necessary to deliver the neck to facilitate engaging the trial head.
The reduction manoeuvre includes removing all retractors from the wound and placing the leg level to the body while traction is applied and the surgeon feels the head as it engages the rim of the acetabular cup. This should be followed by internally rotating the leg by 20-degrees with a gentle downward and distal directed force by the surgeon to reduce the head into the socket. Fluoroscopy should be used at this point to verify the trial stem position and adequacy together with leg length and offset. At this point, it is also possible to affirm the neck cut length as well.
Once offset and leg length are satisfactory, the hip is then dislocated using a sharp hook around the neck anteriorly with upward traction of the hook combined with simultaneous traction. The head and neck trials are removed and all retractors are replaced around the femur in order to remove the trial stem and insert the final femoral stem and head followed hip reduction. When cementing a femoral stem, the cement restrictor can be introduced after the trial component has been removed and the canal irrigated and dried. Commonly used is the combination of a flexible introducer for the cement restrictor together with a flexible brush and special suction with drying tampon.
When addressing a PFF, this approach can be extended in both proximal and distal directions (10). Proximally, the TFL can be released from the iliac crest extending posterior to the ASIS and this can be easily reattached at the end of the procedure, distally the incision can be extended using a “lazy S” incision combined with elevation of Vastus Lateralis and retracting it medially to expose the femur safely (9,10). This extension divides the ITB superficially and utilises the plane between Vastus Lateralis (Femoral nerve) and the Long Head of the Biceps Femoris (Sciatic nerve) deeply (6). The same approach can be used in revision cases that can be combined with anterior osteotomy window to remove the implanted well integrated stem (6,9). This extended version of the approach has been successfully used to perform two stage revision arthroplasty for PPI with good eradication rate and comparable complication rate to other approaches (11).
For closure, the capsule is usually closed by approximating the two capsular leaflets using the number 2-ethibond stay sutures to be followed by tying a surgical knot. In certain occasions especially in revision cases, there is no capsule to close due to prior capsulectomy. The wound is then closed with continuous number 1 vicryl for the fascia in a running locking fashion followed by interrupted 2-0 vicryl for the subcutaneous tissue to be followed by staples and sometimes 3-0 monocryl for the skin.
Conclusions
A comprehensive description of the DAA to the hip for THA using a traction table that can be used as a guide for any surgeon wanting to adopt this approach for THA was presented. This approach is safe to use for primary and revision THA with comparable results to other surgical approaches.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at http://dx.doi.org/10.21037/aoj-20-71
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-71). JH reports that he receives personal fees and other from Stryker, grants, personal fees and other from DePuy, personal fees and other from Smith and Nephew, other from Zimmer, other from Microport, personal fees from Intellijoint, outside the submitted work. BL reports that he receives grants, personal fees and other from Smith and Nephew, grants, personal fees and other from DePuy, grants, personal fees and other from Stryker, other from Zimmer, personal fees from IntelliJoint, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this study and any accompanying images. All the images in this article are original with a written consent for publication.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petis S, Howard JL, Lanting BL, et al. Surgical approach in primary total hip arthroplasty: Anatomy, technique and clinical outcomes. Can J Surg 2015;58:128-39. [Crossref] [PubMed]
- Thaler M, Lechner R, Putzer D, et al. Two-year gait analysis controls of the minimally invasive total hip arthroplasty by the direct anterior approach. Clin Biomech (Bristol, Avon) 2018;58:34-8. [Crossref] [PubMed]
- Mayr E, Nogler M, Benedetti MG, et al. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: A gait analysis study. Clin Biomech (Bristol, Avon) 2009;24:812-8. [Crossref] [PubMed]
- Zomar BO, Bryant D, Hunter S, et al. A randomised trial comparing spatio-temporal gait parameters after total hip arthroplasty between the direct anterior and direct lateral surgical approaches. Hip Int 2018;28:478-84. [Crossref] [PubMed]
- Galakatos GR. Direct anterior total hip arthroplasty. Mo Med 2018;115:537-41. [PubMed]
- Masonis JL, Alden KJ. Extensile Femoral Exposure/Femoral Revision Techniques Via the Direct Anterior Approach. In: Instructional Course Lectures. American Academy of Orthopaedic Surgeons 2020:3-14.
- Light TR, Keggi KJ. Anterior approach to hip arthroplasty. Clin Orthop Relat Res 1980;255-60. [PubMed]
- Watts CD, Houdek MT, Wagner ER, et al. High Risk of Wound Complications Following Direct Anterior Total Hip Arthroplasty in Obese Patients. J Arthroplasty 2015;30:2296-8. [Crossref] [PubMed]
- Nogler MM, Thaler MR. The Direct Anterior Approach for Hip Revision: Accessing the Entire Femoral Diaphysis Without Endangering the Nerve Supply. J Arthroplasty 2017;32:510-4. [Crossref] [PubMed]
- Thaler M, Dammerer D, Krismer M, et al. Extension of the Direct Anterior Approach for the Treatment of Periprosthetic Femoral Fractures. J Arthroplasty 2019;34:2449-53. [Crossref] [PubMed]
- Thaler M, Lechner R, Dammerer D, et al. The direct anterior approach: treating periprosthetic joint infection of the hip using two-stage revision arthroplasty. Arch Orthop Trauma Surg 2020;140:255-62. [Crossref] [PubMed]
- Lanting BA, Odum SM, Cope RP, et al. Incidence of Perioperative Events in Single Setting Bilateral Direct Anterior Approach Total Hip Arthroplasty. J Arthroplasty 2015;30:465-7. [Crossref] [PubMed]
- Martin GR, Marsh JD, Vasarhelyi EM, et al. A cost analysis of single-stage bilateral versus two-stage direct anterior total hip arthroplasty. Hip Int 2016;26:15-9. [Crossref] [PubMed]
- Nizam I, Batra AV. One-stage bilateral anterior bikini total hip replacement − experience of two cases. Sicot J 2018;4:35. [Crossref] [PubMed]
- Petis SM, Howard JL, Lanting BA, et al. In-Hospital Cost Analysis of Total Hip Arthroplasty: Does Surgical Approach Matter? J Arthroplasty 2016;31:53-8. [Crossref] [PubMed]
- Grammatopoulos G, Gofton W, Cochran M, et al. Pelvic positioning in the supine position leads to more consistent orientation of the acetabular component after total hip arthroplasty. Bone Joint J 2018;100-B:1280-8. [Crossref] [PubMed]
- Zomar BO, Bryant D, Hunter S, et al. The effect of conjoint tendon release on gait after direct anterior total hip arthroplasty. Hip Int 2019;29:578-83. [Crossref] [PubMed]
- Petis SM, Vasarhelyi EM, Howard JL, et al. Gait analysis following release of the short external rotators during an anterior approach for total hip arthroplasty. Hip Int 2018;28:584-90. [Crossref] [PubMed]
- Yao R, Howard JL, Lanting BA. Conjoint Tendon Release in Direct Anterior Total Hip Arthroplasty: No Impact on Patient Outcomes. Orthopedics 2017;40:e971-4. [Crossref] [PubMed]
- Eto S, Hwang K, Huddleston JI, et al. The Direct Anterior Approach is Associated With Early Revision Total Hip Arthroplasty. J Arthroplasty 2017;32:1001-5. [Crossref] [PubMed]
- Rivera F, Leonardi F, Evangelista A, et al. Risk of stem undersizing with direct anterior approach for total hip arthroplasty. Hip Int 2016;26:249-53. [Crossref] [PubMed]
Cite this article as: Ibrahim M, Thompson J, Howard J, Lanting B. The direct anterior approach to the hip for total hip arthroplasty: a blind guide (with traction table). Ann Joint 2021;6:33.