
Osteosarcoma mineralization changes on radiographs have moderate correlation to chemotherapy response using bone subtraction methodology
Introduction
Osteosarcoma is the most common primary bone cancer in young people (1). Treatment usually involves preoperative chemotherapy, resection of the primary tumor, and postoperative chemotherapy. Survival from osteosarcoma is correlated with chemotherapy response, determined by tumoral necrosis, described by Huvos and Picci (2-4). By the Huvos criteria, grade 1 equates to <50% necrosis, grade 2 is 50–90%, grade 3 is 90–99%, grade 4 is 100% necrosis; a good response is considered Huvos grade 3 or 4. Unfortunately, the response to chemotherapy is unknown until the primary tumor is analyzed following resection, potentially subjecting patients with osteosarcoma to weeks of ineffective, cytotoxic treatment. Currently, there is no non-invasive and low-cost method of measuring the response to chemotherapy.
Radiological imaging is a key element in the identification, diagnosis, and staging of an osteosarcoma tumor. Osteosarcomas incur predictable features on plain radiographs, generally showing a wide zone of transition, an osseous matrix, and aggressive periosteal reactions with a soft-tissue mass (5,6). Magnetic resonance imaging (MRI), computed tomography (CT), and 99Tecnetium bone scan are required elements of osteosarcoma staging, providing critical information about the presence of intramedullary skip metastases and distant bony or pulmonary metastases (7). A long-recognized phenomenon occurring commonly in the treatment of patients with osteosarcoma is a perceived increase in the primary tumor’s mineralization during chemotherapy (Figure 1) (8). Prior investigators—hypothesizing that increased mineralization portends a better response to chemotherapy—attempted unsuccessfully to correlate this mineralization change and chemotherapy effect using subjective, visual interpretation of tumor mineralization (8-10). These earlier methods did not allow for computer-assisted grayscale normalization and relied on the human eye’s minimum noticeable change in input intensity—the so-called “increment threshold”—which is known to be inexact and unreliable for work that requires high-fidelity grayscale measurement (11). Since this work, sophisticated image analysis tools were developed and, to date, no investigators have investigated if mineralization changes are associated with chemotherapy response using these tools.
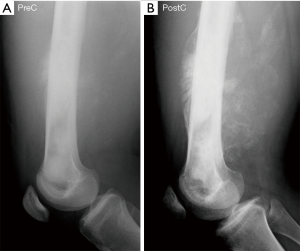
This investigation was a retrospective review of radiographs obtained before and after the administration of chemotherapy for patients with lower extremity osteosarcoma. The primary research aim was to analyze the association between tumor mineralization and chemotherapy response using computer-assisted image analysis techniques. The primary hypothesis was that an increase in mineralization is associated with higher necrosis. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/aoj-20-70).
Methods
Patient data
We conducted database inquiries for patients with a conventional, high-grade osteosarcoma diagnosis who had pre- (PreC) and post-chemotherapy (PostC) radiographs of the primary tumor from lateral side obtained prior to resection. All patients were prescribed a 3-month course of chemotherapy—doxorubicin, methotrexate, and cisplatin as general, first-line therapy (12,13)—prior to their definitive tumor resection. Following surgery, tumor necrosis values, determined originally by histological review with attention to criteria described by Picci (4), were obtained via chart review.
Thirty-one patients met criteria, their treatments were performed between 1999 and 2013. There were 16 (51%) males and 15 (49%) females; mean age at the treatment initiation was 13 years (range, 4 to 20 years). Anatomical locations of the primary tumors included two proximal femoral tumors, twenty distal femoral tumors, seven proximal tibial tumors, and two distal tibial tumors.
This research was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Dartmouth-Hitchcock Medical Center Committee for the Protection of Human Subjects, Study #00027973. Informed consent was not required for this retrospective, non-interventional study, which was considered minimal risk.
Radiographs
All patients had PreC and PostC radiographs of the primary tumors. Based upon availability, radiographs were digitized copies of plain film radiographs. Because of greater variability in the AP radiographs’ field of view and limb alignment, we confined this analysis to lateral radiographs only.
Image analysis
Digitized radiographs were analyzed using MATLAB (v. 2018b, MathWorks, Natick, MA, USA) by a reviewer blinded to tumor necrosis values. The ROIs were identified by an orthopaedic oncologist by tracing the periphery of all tumors on the PostC lateral radiographs; regions of interest (ROIs) of normal bone were also identified. Radiograph luminance in the ROIs was considered a surrogate for radiodensity. To compensate for the difference in scanning quality, all images with different bit depth were normalized on a scale of 0–1 with arbitrary units (AU). Each patient’s PreC and PostC images were co-registered by identifying identical anatomical landmarks in both images. Anatomical landmarks were identified using the validated and open-source software extension fitgeotrans.m. These landmarks were processed to provide co-registration between the PreC and PostC images, reconciling differences in the images based on limb position. The ROIs defined on the PostC images were reproduced on the co-registered PreC images to provide an exact comparison. The accuracy of the co-registered PreC and PostC images was measured using another validated and open-source software extension imshowpair.m.
Following image co-registration and ROI definition, PreC and PostC images were normalized to the non-tumor-affected bone ROI luminance values. Tumor mineralization change was determined by subtracting the average luminance of the PreC tumor ROI from the PostC tumor ROI. The tumor mineralization change was calculated using Equation.1, with T indicating mean tumor luminance and B indicating mean bone luminance.
Tumor mineralization = (PostC-T − PostC-B) − (PreC-T − PreC-B)
Statistical analysis
The relationship between luminosity-defined mineralization changes and osteosarcoma necrosis was determined using Pearson’s correlation coefficient (ρ) and coefficient of determination (R2).
Results
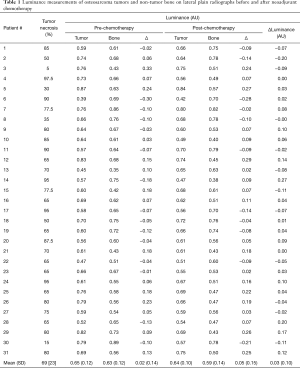
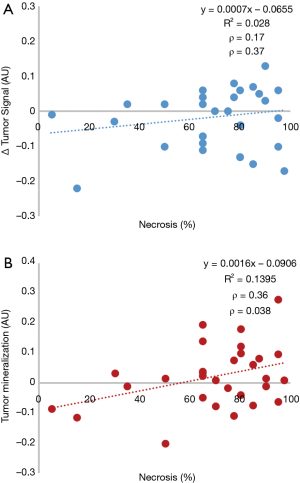
The mean osteosarcoma tumor necrosis value for this study was 69%±23% (Table 1). Mean luminance values for PreC and PostC non-tumor-affected bone were 0.63±0.12 and 0.59±0.14, respectively. Mean luminance values for PreC and PostC tumor were 0.65±0.12 and 0.64±0.10, respectively. The direct value difference of PreC and PostC showed a low correlation—Pearson correlation coefficient (ρ) of 0.17 (P=0.37)—with the tumor necrosis value (Figure 2A). Osteosarcoma mineralization change calculated based on Eq. [1] showed moderate correlation—Pearson correlation coefficient (ρ) of 0.36 (P=0.038)—with the tumor necrosis value (Figure 2B).
Full table
Discussion
Given the importance of chemotherapy response to oncological outcomes of patients with osteosarcoma, a low-cost and low-harm method of assessing this endpoint would likely prove beneficial in maximizing survival. Noting the subjective observation that osteosarcoma tumors with good chemotherapy response often appear to demonstrate increased mineralization during treatment, previous authors were unable to identify a significant correlation between mineralization and treatment response using qualitative methods (8,10,14). Through software-based review of radiographs obtained PreC and PostC, we were able to demonstrate a modest positive correlation between tumor mineralization and chemotherapy response, as determined by tumor necrosis.
Smith first described seeing “striking and unusual radiographic changes” in osteosarcoma tumors during chemotherapy (8). They described a statistically unsupported positive correlation between the presence of these changes and osteosarcoma necrosis. Similarly, Hirano described a trend toward higher radiographic density in patients with osteosarcoma and malignant fibrous histiocytoma of bone with better chemotherapy response, however, their study was underpowered to demonstrate significance (9). Holscher used a three-tiered, human-scored rating system to gauge mineralization changes during chemotherapy (increased, unchanged, or decreased) (10). Their methods did not allow for radiograph normalization and relied on the human eye’s minimum noticeable change in input intensity—the so-called “increment threshold”—which is known to be inexact (11), for evaluating differences in radiograph luminance; they found no correlation between mineralization and treatment response. Lindner developed a four-tiered system of evaluating local host response on histological examination and found significant correlation both to tumor necrosis and to mineralization on CT (14). They were unable, however, to demonstrate a correlation to changes on radiographs. Because conventional radiography and CT are similar technologies that sample identical tissues, it is intuitive therefore that the radiography data contains all the contents of CT, just unclarified in the axial plane by surrounding tissues. Therefore, it is plausible that, with adequate normalization and subtraction techniques, radiography data may be distilled to provide the granularity of CT without the additional radiation exposure and cost.
Other imaging modalities have been investigated for their ability to assess chemotherapy response in osteosarcoma. MRI has been researched by multiple research teams, all concluding that the apparent diffusion coefficient is of potential value in grading chemotherapy response (15-17). 99Technetium and 201Thallium scintigraphy also appear to have predictive validity in evaluating chemotherapy response, although they have received less attention than MRI (18,19). Positron-emission computed tomography with 18fluorodeoxygluose also demonstrates predictive validity in assessing chemotherapy response though there is higher cost and radiation exposure compared to plain radiography (20-22).
This study has limitations. Radiographs analyzed in this study were of limited numbers (31 patients from two centers) and obtained via heterogeneous techniques and were often scanned copies of film radiographs. We acknowledge the deleterious effects that these study flaws have on the ability to draw definitive conclusions from our data. We were unable to account for the imager settings—kilovoltage peak and milliamp seconds—and the source-to-image distance used to obtain the radiographs. We also limited our analysis for this study to lateral radiographs, which we found had greater technique homogeneity—in further validation studies we would pursue tumor assessment in anteroposterior and lateral radiographs. Another limitation was our image normalization technique, by which we normalized images using the luminance of bone not affected by tumor. This method assumes that bone radiodensity is unchanged during the treatment process, which may not be true. Despite these limitations, we believe that rather than serve as a definitive and conclusive study, these early findings bolster the need to further examine whether osteosarcoma mineralization changes may provide low-cost, low-radiation, and non-invasive guidance regarding treatment effect.
In conclusion, changes in osteosarcoma mineralization on plain lateral radiographs show moderate correlation with chemotherapy response when analyzed with sophisticated software tools using bone subtraction methodology. Our next step is to pursue funding to support work to determine: (I) are these findings supported, enhanced, or refuted when using state-of-the-art, high-resolution digital radiographs obtained with uniform technique and phantoms for image normalization; (II) how does the accuracy of this method, when optimized, compare to CT or dual-energy radiography; (III) how early in neoadjuvant chemotherapy could treatment response potentially be determined? Demonstration of a reliable correlation between tumor radiodensity changes and chemotherapy response may enable earlier recognition of treatment failure via analysis of low-cost, low-radiation, and standard-of-care plain radiographs.
Acknowledgments
Funding: Dr. Henderson is funded by NIH-NIBIB 1K23EB026507.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Kurt R. Weiss and Mitchell S. Fourman) for the series “Osteosarcoma” published in Annals of Joint. The article has undergone external peer review.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/aoj-20-70
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aoj-20-70
Peer Review File: Available at http://dx.doi.org/10.21037/aoj-20-70
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-70). The series “Osteosarcoma” was commissioned by the editorial office without any funding or sponsorship. ERH serves as an unpaid editorial board member of Annals of Joint from Sep 2018 to Aug 2020. ERH reports grants from NIH-NIBIB, during the conduct of the study; personal fees from Stryker Orthopaedics, grants from NIH-NCI, grants from Orthopaedic Research and Education Foundation, grants from Hitchcock Foundation, outside the submitted work. KSS reports grants from NCI (R37CA212187, R01 CA184354) and Mark Foundation for Cancer Research, outside the submitted work. BWP reports grants from NIH-NCI grant#NCI (R01 CA109558, R01 CA167413, R01 CA192803, P01 CA084203), outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This research was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Dartmouth-Hitchcock Medical Center Committee for the Protection of Human Subjects, Study #00027973. Informed consent was not required for this retrospective, non-interventional study, which was considered minimal risk.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jones LB, Barr JS. Bone sarcomas: an update of the recent literature. Curr Orthopaed Prac 2016;27:582-6. [Crossref]
- Friebele JC, Peck J, Pan X, et al. Osteosarcoma: A Meta-Analysis and Review of the Literature. Am J Orthop (Belle Mead NJ) 2015;44:547-53. [PubMed]
- Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med 1977;101:14-8. [PubMed]
- Picci P, Bacci G, Campanacci M, et al. Histologic evaluation of necrosis in osteosarcoma induced by chemotherapy. Regional mapping of viable and nonviable tumor. Cancer 1985;56:1515-21. [Crossref] [PubMed]
- Caracciolo JT, Temple HT, Letson GD, et al. A Modified Lodwick-Madewell Grading System for the Evaluation of Lytic Bone Lesions. AJR Am J Roentgenol 2016;207:150-6. [Crossref] [PubMed]
- Lodwick GS, Wilson A, Farrell C, et al. Determining growth rates of focal lesions of bone from radiographs. Radiology 1980;134:577-83. [Crossref] [PubMed]
- Eftekhari F. Imaging assessment of osteosarcoma in childhood and adolescence: diagnosis, staging, and evaluating response to chemotherapy. Cancer Treat Res 2009;152:33-62. [Crossref] [PubMed]
- Smith J, Heelan RT, Huvos AG, et al. Radiographic changes in primary osteogenic sarcoma following intensive chemotherapy. Radiological-pathological correlation in 63 patients. Radiology 1982;143:355-60. [Crossref] [PubMed]
- Hirano T, Iwasaki K, Kumashiro T, et al. Encapsulation around malignant bone tumors after preoperative adjuvant treatment. Nihon Seikeigeka Gakkai Zasshi 1992;66:31-7. [PubMed]
- Holscher HC, Hermans J, Nooy MA, et al. Can conventional radiographs be used to monitor the effect of neoadjuvant chemotherapy in patients with osteogenic sarcoma? Skeletal Radiol 1996;25:19-24. [Crossref] [PubMed]
- Meese TS, Georgeson MA, Baker DH. Binocular contrast vision at and above threshold. J Vis 2006;6:1224-43. [Crossref] [PubMed]
- Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol 2000;18:4016-27. [Crossref] [PubMed]
- Souhami RL, Craft AW, Van der Eijken JW, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet 1997;350:911-7. [Crossref] [PubMed]
- Lindner NJ, Scarborough MT, Spanier SS, et al. Local host response in osteosarcoma after chemotherapy referred to radiographs, CT, tumour necrosis and patient survival. J Cancer Res Clin Oncol 1998;124:575-80. [Crossref] [PubMed]
- Hayashida Y, Yakushiji T, Awai K, et al. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: initial results. Eur Radiol 2006;16:2637-43. [Crossref] [PubMed]
- Oka K, Yakushiji T, Sato H, et al. The value of diffusion-weighted imaging for monitoring the chemotherapeutic response of osteosarcoma: a comparison between average apparent diffusion coefficient and minimum apparent diffusion coefficient. Skeletal Radiol 2010;39:141-6. [Crossref] [PubMed]
- Uhl M, Saueressig U, Koehler G, et al. Evaluation of tumour necrosis during chemotherapy with diffusion-weighted MR imaging: preliminary results in osteosarcomas. Pediat Radiol 2006;36:1306-11. [Crossref] [PubMed]
- Kobayashi Y, Ozaki T, Takeda Y, et al. Evaluation of the effect of preoperative chemotherapy in bone sarcomas:99mTc-HMDP scintigraphy in 34 cases. Acta Orthop Scand 1998;69:611-6. [Crossref] [PubMed]
- Kunisada T, Ozaki T, Kawai A, et al. Imaging assessment of the responses of osteosarcoma patients to preoperative chemotherapy: angiography compared with thallium-201 scintigraphy. Cancer 1999;86:949-56. [Crossref] [PubMed]
- Benz MR, Czernin J, Tap WD, et al. FDG-PET/CT Imaging Predicts Histopathologic Treatment Responses after Neoadjuvant Therapy in Adult Primary Bone Sarcomas. Sarcoma 2010;2010:143540 [Crossref] [PubMed]
- Byun BH, Kong CB, Lim I, et al. Combination of 18F-FDG PET/CT and diffusion-weighted MR imaging as a predictor of histologic response to neoadjuvant chemotherapy: preliminary results in osteosarcoma. J Nucl Med 2013;54:1053-9. [Crossref] [PubMed]
- Byun BH, Kong CB, Lim I, et al. Early Response Monitoring to Neoadjuvant Chemotherapy in Osteosarcoma Using Sequential 18F-FDG PET/CT and MRI. Eur J Nucl Med Mol Imaging 2014;41:1553-62. [Crossref] [PubMed]
Cite this article as: Henderson ER, Xu X, Pogue BW, Samkoe KS, Anderson ME. Osteosarcoma mineralization changes on radiographs have moderate correlation to chemotherapy response using bone subtraction methodology. Ann Joint 2020;5:38.


