
Hip resurfacing: is female gender an absolute or relative contraindication?
Introduction
In the young arthritic hip, female gender should not be a contraindication to metal on metal (MoM) hip resurfacing arthroplasty (HRA) (1) to the extent that the author would argue that, resurfacing is a superior option to standard stemmed total hip replacement (THR) in these patients. Starting in the early years of MoM HRA an attempt was made to identify causes for failures in hip resurfacing (2). Over the years numerous risk factors including female gender, dysplasia, osteonecrosis, small bearing size, femoral head cysts, and advanced age have been linked to a higher risk for failure. This has led to a practice of patient selection where only young men with osteoarthritis (OA) and strong bone are left as good candidates for resurfacing. This process was driven by two unproven assumptions; first, that a person that was identified as higher risk for resurfacing would have a better outcome with standard THR, and second, that implant survivorship is the only important factor that should drive the choice between HRA and THR. Both assumptions are somewhat biased, therefore, a reevaluation of the way we use “risk factors” in the context of MoM hip resurfacing is important. Risk factors should appropriately be used to inform patients of their risks for surgery, especially if they are factors such as obesity or smoking that the patient could choose to modify prior to undertaking elective surgery. Also, they can simply be used for accurate informed consent. However, if it is proposed that risk factors should be used to deselect a patient from hip resurfacing, it should require that the alternative solution to their severe hip arthritis, namely THR, is a better option when all outcome measures are considered. Finally, risk factors can serve to focus our attention on the problems in HRA so that we can develop solutions for them and thereby improve the overall success of the operation.
After 25 years of modern MoM hip resurfacing, it has now become clear that HRA is not only more durable (3) but also more functional (4,5) than THR in the young patient. It preserves femoral bone stock for the unlikely need for revision and it has even been shown to improve patient survivorship at 10 years (6,7). However, because of the lack of surgeons adequately trained to perform hip resurfacing there is a continued perception that its performance is inferior to THR which rightfully has been named the “operation of the (20th) century” (8). But one could ask: will it still retain its title in the 21st century? Surely patients want the longest lasting implants, but they also may desire a hip that allows walking without thigh pain, walking at a fast pace without a limp, even running and impact sports (4,9). They surely desire a hip that doesn’t dislocate and that does not require them to avoid certain movements. Almost certainly they would choose a hip that adds years to their life (6,7). Patients seem to understand better than many surgeons that a better outcome can be achieved with hip arthroplasty if we perform an operation that more closely resembles the natural hip, an operation that reproduces natural hip mechanics more closely. It is a general principle of orthopedics that a reconstruction has a better chance of success if it more closely mimics nature. After 25 years of MoM resurfacing, we have come to the point where evidence supports this intuition.
Registry data/implant survivorship
Registry data is often used to compare the outcome of HRA to THR where Smith (10) concluded that HRA was inferior based on 5-year implant survivorship data from the British Registry. There are numerous limitations to this study. The first is that there was no adjustment for surgeon skill and experience. The second is that the British Registry has only an 80–90% capture rate (11). Finally, the only factor that is considered to reach the sweeping conclusion that HRA leads to inferior outcomes is implant survivorship. But implant survivorship is only one factor in the overall assessment of an intervention’s effectiveness. Registry data can be used as a very crude assessment of average surgeon results and cannot account for varying levels of surgeon skill and experience creating a considerable bias when THR and HRA are compared in a registry. THR has been performed routinely since the 1960s; all orthopedic surgeons are taught how to perform this operation with some proficiency during their training whereas the current generation of MoM HRA come from the two surgeons: McMinn (12) and Amstutz (13). In the paper by Smith, there is no mention that the average surgeon volume for HRA in the registry was 2.6 cases/year where it is certain that those same surgeons performed THRs at a much greater volume and longstanding experience. It seems that the proper conclusion to this paper should have been: “in the hands of surgeons inexperienced in hip resurfacing, implant survivorship is better with THR”; more extensive training for surgeons wanting to perform HRA may be required.
Recently an international study group has been formed to address this problem (14). Twenty-eight dedicated resurfacing surgeons from 13 countries have pooled their data including all of their “learning curve cases”. We have analyzed up to 22-year Kaplan-Meier implant survivorship using 6 different implants in 11,066 patients under 50 years age. This age cut off has been chosen to emphasize the durability of HRA when compared to THR. THR has reasonably good implant survivorship in older patients, but not in younger patients. The Swedish register indicates only an 83% 10-year (15) and 54% 20-year survivorship in patients under 50; similar in men and women. In this large multicenter study HRA is demonstrated to have 95% implant survivorship at 10 years and 90% at 20 years. At 20 years implant survivorship is better in men at 93%, but women still fare better with HRA than THR with survivorship rates of 81% compared to 52% (14,16-18).
This certainly has been my experience over the last twenty years. My first cohort of hybrid MoM resurfacing in young patients, 10-year implant survival was better than expected at 89% (19) and my latest 12-year Kaplan-Meier survivorship in over 5,000 uncemented HRA now stands at 99% (20).
Failures and improvements in resurfacing in women
Rather than use risk factor analysis to deselect patients from hip resurfacing, I have used risk factor analysis to focus new strategies on improving the outcome of higher-risk patients. This has benefitted all patients, particularly women. In this section, I will explain how this has been accomplished.
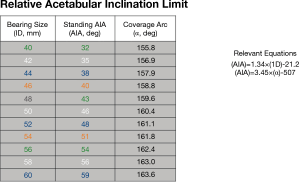
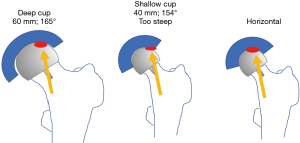
Adverse wear related failure (AWRF) occurs more frequently in patients with small implant bearing sizes. Women tend to require smaller implant sizes; thus, women are at higher risk for AWRF. Some have promoted metal allergy as the cause without providing any convincing evidence for this hypothesis (21). The cause for AWRF has subsequently been shown to be caused by edge loading wear patterns (22,23). Edge loading occurs when acetabular implants have a low coverage arc and/or when surgeons place them too steeply or too anteverted. In my experience, this is most likely to occur in a patient that exhibits a large amount of posterior pelvic tilt on standing radiographs (24). Acetabular components of hip resurfacing implants have a sub-hemispherical coverage arc mimicking the natural acetabulum. It is believed that an 180° coverage arc (as in most THR) would result in impingement with the retained large natural femoral neck. But coverage arc is not the same for all implants. By a quirk of design; coverage arc increases as implant bearing size increases. For example, coverage arc in the Biomet Magnum cup, which I use, varies from 156° in bearing size 40 mm up to 164° in bearing size 60 mm (Figure 1). Therefore, a smaller implant with a lower coverage arc is more likely to develop edge loading and therefore AWRF at any given acetabular inclination angle (AIA) measured on a standing radiograph. Every implant develops a so-called “contact patch” (25) that can be seen on specialized imaging (but not by the naked eye) on retrieved implants. If this primary wear area region is sufficiently separated from the edge of the acetabular component, a fluid film is maintained and unlimited low wear results. If the socket is implanted such that the contact patch is too close to the edge, the fluid film cannot be maintained and a high wear state termed “edge-loading” is created. Ion levels become elevated and eventually enough cobalt and chrome debris get deposited in the tissues surrounding the hip to create a painful inflammatory condition called AWRF (26). Components that are placed to steeply and too anteverted (27) on standing radiographs are more likely to result in edge loading. In an analysis of over 700 cases with standing radiographs and blood ion levels we discovered a “safe zone” (Figure 1) where AWRF is never seen (28). The relative acetabular inclination limit (RAIL) is a straight line that relates inclination angle on the Y-axis to bearing size on the X-axis. It represents the maximum “safe” AIA for any specific bearing size. If the AIA of the component, as measured on a standing radiograph, is below RAIL, then edge loading will not occur. The only exception to this rule is if anteversion is extreme. It is not possible to measure anteversion accurately on plane films, therefore RAIL was established without an anteversion component, nevertheless anteversion must be set at ±10 degrees with respect to the transverse acetabular ligament (TAL) for RAIL to be effective (–10° version wrt TAL, used in cases of extreme posterior pelvic tilt, does NOT result in radiographic retroversion). In 90% of cases, the TAL can be visualized in surgery, in the remainder, the teardrop is used as a substitute. The AIA is measured in the operating room on a normalized to standing intraoperative radiograph (NSIOR). At the same time, anteversion is qualitatively confirmed to be acceptable on this image. We assume that any competent surgeon would not place a component in radiographic retroversion; therefore, any degree of “oval” appearance suggests anteversion. A component that is visualized as “neutral” with very crisp edges to one that is slightly oval is acceptable (Langton Grade A or B) (29). If the component appears very oval and distinct edges cannot be found, then anteversion is excessive. If a component does not meet RAIL or is too anteverted on the NSIOR it is repositioned until the X-ray verifies correct positioning. In a subsequent series of 2,466, we have demonstrated that 100% meet the RAIL criteria and none fail due to AWRF if this protocol is followed (30). In summary, shallower components must be placed more horizontally to avoid edge-loading and subsequent AWRF (Figure 2). Based on this evidence we can confidently assert that the problem of AWRF in MoM HRA has been overcome (31).
Failure of acetabular fixation (FAF) is more common in patients with significant acetabular deformity. This may occur in cases of end stage OA with superior segmental defects due to bone erosion, post-traumatic OA due to acetabular fractures or in Legg-Perthes or dysplasia which both have shallow oval sockets. FAF should be distinguished from late acetabular loosening (LAL) of an initially bone ingrown component due to debonding (32) of the porous coating from the implant substrate or debris mediated loosening common in old style cemented THR sockets. We define FAF as all socket fixation failures that are diagnosed before 2 years or ones that are diagnosed later if they were symptomatic before 2 years.
This discussion will focus on dysplasia because it is the more common diagnosis to lead to FAF and it is much more common in women (90% of dysplasia cases are women); it is present in approximately 30% of young women seeking HRA. Initially only components without supplemental fixation were available, resulting in a high failure rate in women with very oval sockets (33). A related problem in these small shallow oval sockets is that surgeons excessively anteverted and inclined the component to try to maximize component coverage. We now understand that this places the acetabular component at risk for edge-loading and subsequent AWRF (28). I usually placed the component more horizontal and parallel to the TAL, which left the anterior superior edge less covered and led to a higher risk of FAF. Because of this I had relatively fewer AWRF but more FAF. In 2007 a component with supplemental “Trispike” fixation became available. When this was used in all cases where trial component orientation indicated estimated wall uncoverage of greater than 30%, all FAF were subsequently eliminated in this high-risk cohort (33).
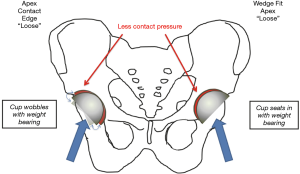
In combination, better fixation (avoiding FAF) and better orientation of the acetabular component (avoiding AWRF) were the main factors that improved implant survivorship in dysplasia from 89% at 8 years to our current 99% at 12 years (34). Not only does this equalize the durability of HRA in women and men, it demonstrates better survivorship with HRA than THR for dysplasia (35). Finally, a wedge-fit acetabular preparation technique has been developed to eliminate all remaining cases of FAF (36) (Figure 3).
LAL describes an acetabular component that initially achieves bone in growth, but then loses fixation after 2 years. It is a rare problem that could occur in long neglected cases of AWRF where extensive amount of debris is allowed to accumulate locally and cause lysis (26). This did occasionally occur in the early days when AWRF was poorly understood. The primary cause of failure would then be ascribed to AWRF. Another cause we have seen is when an initially well-fixed acetabular component later becomes loose by the process of debonding of a titanium porous coating from a cobalt chrome substrate in the Corin Cormet 2000 brand (32) which is no longer on the market.
Femoral failure. Early femoral failure (EFF) (including femoral neck fracture within 6 months and femoral head collapse within 2 years) has been seen more commonly in patients of older age and female gender. Our multivariate analysis found that these demographic factors were dependent variables (37). Bone density of the femoral neck and BMI above 29 proved to be the only independent risk factors. In other words, advanced age and female gender do not need to be considered as a risk factor for femoral neck fracture if bone density is known. More importantly, we were able to use this knowledge to develop modified postoperative management programs for at-risk patients consisting of longer weight bearing protection and anti-resorptive agents that we have since shown to decrease the EFF rate from 2% to below 0.1% (38). Femoral head cysts were identified early on as a risk factor (2) where cement was used to fill in the defect. By using cementless fixation and filling the defect with bone graft, head cysts up to 3 cm3 did not result in a higher failure rate (39). Similarly, late femoral loosening at 12 years follow-up has been reduced from 1.1% to 0% with the adoption of uncemented femoral components (20) (Figure 4).

Infection is the worst implant related complication that can occur to any joint replacement patient. This discussion is limited to perioperative infections (because the surgeon has control over these) which I define as any deep infection diagnosed before 6 months or any later diagnosis of deep infection in which the patient became symptomatic before 6 months. In the Medicare database a 3-month deep infection rate of 1% is probably an underestimate of the true rate of deep perioperative infection. Others have indicated 2–3% as the benchmark. My current 6-month deep infection rate is 0.4% in 3,400 cases with none requiring implant removal (20).
Unexplained pain can be a cause for dissatisfaction after hip arthroplasty and sometimes leads to revision with unclear benefit. Hip arthroplasty never creates a normal hip and therefore some degree of unexplained pain is to be expected. In retrospect, some patients did not have severe enough cartilage damage preoperatively to warrant THR. Pain is subjective. Twenty percent of asymptomatic HRA and THR have a small amount of fluid collection on MARS MRI (40). If a patient is symptomatic and has a fluid collection… is this AWRF or trunnion corrosion? This is a most difficult problem. Unfortunately, the indiscriminate bias of hip arthroplasty surgeons against metal bearings has led to ill-advised revisions of MoM HRA. Residual unexplained pain is also relative to patient activity goals. Most patients describe themselves as “active”. An “active” older patient with a THR who wants to golf and play with their grandchild may have no pain, but a younger patient who wants to be “active” and play impact sports with the same implant will be unable probably due to thigh pain. Age matched HRA patients have more normal maximal gait patterns and are more likely to resume impact sports (5). We have some data to suggest that improvements in acetabular preparation have led to a lower incidence of unexplained residual pain in HRA (36) possibly by increasing the incidence of bone ingrowth at the expense of stable fibrous ingrowth. Also, efforts at reducing psoas tendonitis by avoiding anterior-inferior cup edge overhang may have contributed. Dissatisfaction and residual pain on Harris Hip Score occurs in approximately 10–20% of THR (41,42), residual moderate pain is currently present in only 2% in my HRA (36).
Dislocation is caused by cutting the major hip ligaments and reconstructing the hip with abnormal biomechanics. Because HRA retains a normal bearing size and femoral offset HRA carries a very low 0.3% risk of early dislocation and a 0.1% rate of revision for recurrent instability (43). This remains true even for high-risk women with dysplasia. There has been no change in this failure mode during the last 20 years. Dislocation risk in THR is substantially higher (44).
Key components to avoid failure in females undergoing HRA
- AWRF has been reduced from 1% at 10 years to 0% at 8 years by a better understanding of its cause, development of a “safe zone” that can be achieved in 100% of cases by using the technique of NSIOR.
- FAF has been reduced to zero in 12 years in dysplasia and from 0.5% overall to 0.1% at 6 years by selectively employing supplemental “Trispike” fixation if the component is uncovered by ≥30% and implementing a wedge-fit preparation technique for all components.
- EFF has been reduced from 2% to 0.1% using risk stratification by bone density and BMI and employing bisphosphonates and initial weight bearing restrictions in higher risk groups.
- Femoral head cysts up to 3 cm3 do not carry a higher risk as long as they are not filled with cement. Either bone grafting a cavitary defect and cementing over it or using uncemented fixation works. Segmental defects can be handled easily with bone graft and an uncemented femoral component, but it is hard to avoid excess cement in these.
- Late femoral failure (LFF) has been eliminated by introduction of an uncemented femoral component. We are not certain that it may not also have contributed in some way to the reduction of EFF.
Conclusions
The fact that hip resurfacing is technically more demanding is generally accepted but difficult to demonstrate. Every orthopedic surgeon learns to perform a THR in residency. MoM HRA has been maligned primarily because of failures of MoM THR (45) and because of poor outcomes of HRA in registries (10) where most cases are done by very inexperienced (2–3 cases/year) surgeons. By following the RAIL guideline, metallosis has been overcome. Hip resurfacing is the correct operation for most young patients with premature hip degeneration of any cause. THR is an excellent solution, but by all measures of success: implant survivorship, function, stability, lack of residual unexplained pain, bone preservation and even all-cause mortality, hip resurfacing must be judged as an improvement. Men and women alike have better outcomes with hip resurfacing.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (George Grammatopoulos and Paul E. Beaulé) for the series “Hip Resurfacing for the Young Arthritic Hip” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2020.04.05). The series “Hip Resurfacing for the Young Arthritic Hip” was commissioned by the editorial office without any funding or sponsorship. TPG reports personal fees from Zimmer Biomet, personal fees from Smith Nephew Richards, outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gaillard EB, Gaillard MD, Gross TP. Interventions for improving hip resurfacing outcomes in women: a high-volume, retrospective study. J Arthroplasty 2017;32:3404-11. [Crossref] [PubMed]
- Beaulé PE, Dorey FJ, Le Duff MJ, et al. Risk factors affecting outcome of metal-on-metal surface arthroplasty of the hip. Clin Orthop Relat Res 2004;87-93. [Crossref] [PubMed]
- Gaillard MD, Gross TP. Metal-on-metal hip resurfacing in patients younger than 50 years: a retrospective analysis: 1285 cases, 12-year survivorship. J Orthop Surg Res 2017;12:79. [Crossref] [PubMed]
- Aqil A, Drabu R, Bergmann JH, et al. The gait of patients with one resurfacing and one replacement hip: a single blinded controlled study. Int Orthop 2013;37:795-801. [Crossref] [PubMed]
- Barrack RL, Ruh EL, Berend ME, et al. Do young, active patients perceive advantages after surface replacement compared to cementless total hip arthroplasty? Clin Orthop Relat Res 2013;471:3803-13. [Crossref] [PubMed]
- Kendal AR, Prieto-Alhambra D, Arden NK, et al. Mortality rates at 10 years after metal-on-metal hip resurfacing compared with total hip replacement in England: retrospective cohort analysis of hospital episode statistics. BMJ 2013;347:f6549. [Crossref] [PubMed]
- McMinn DJ, Snell KI, Daniel J, et al. Mortality and implant revision rates of hip arthroplasty in patients with osteoarthritis: registry based cohort study. BMJ 2012;344:e3319 [Crossref] [PubMed]
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007;370:1508-19. [Crossref] [PubMed]
- Barrack RL. Metal-metal hip resurfacing offers advantages over traditional arthroplasty in selected patients. Orthopedics 2007;30:725-6. [Crossref] [PubMed]
- Smith AJ, Dieppe P, Howard PW, et al. Failure rates of metal-on-metal hip resurfacings: analysis of data from the National Joint Registry for England and Wales. Lancet 2012;380:1759-66. [Crossref] [PubMed]
- National Joint Registry: 13th annual report, National Joint Registry for England, Wales and Northern Ireland and the Isle of Man. 2016.
- Cutts S, Carter PB. Hip resurfacing: a technology reborn. Postgrad Med J 2006;82:802-5. [Crossref] [PubMed]
- Amstutz HC, Beaulé PE, Dorey FJ, et al. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am 2004;86:28-39. [Crossref] [PubMed]
- van Der Straeten C. International high-volume centers’ report on the outcome of 11,382 Metal-on-Metal Hip Resurfacings in patients 50 years at surgery. Boston: International Society for Technology in Arthroplasty, 2016.
- Overgaard S, Petersen A, Havelin LI, et al. The prognosis of total hip arthroplasty (THA) in patients younger than 50 years of age, results of 14,610 primary THA. J Bone Joint Surg Br 2011;96:87.
- Pedersen AB, Mehnert F, Havelin LI, et al. Association between fixation technique and revision risk in total hip arthroplasty patients younger than 55 years of age. Results from the Nordic Arthroplasty Register Association. Osteoarthritis Cartilage 2014;22:659-67. [Crossref] [PubMed]
- Kärrholm J, Lindahl H, Malchau H, et al. The Swedish Hip Arthroplasty Register: Annual Report 2016. 2016. Available online: https://registercentrum.blob.core.windows.net/shpr/r/Annual-Report-2016-B1eWEH-mHM.pdf
- Mäkelä KT, Matilainen M, Pulkkinen P, et al. Countrywise results of total hip replacement. An analysis of 438,733 hips based on the Nordic Arthroplasty Register Association database. Acta Orthop 2014;85:107-16. [Crossref] [PubMed]
- Gross TP, Liu F, Webb LA. Clinical outcome of the metal-on-metal hybrid Corin Cormet 2000 hip resurfacing system: an up to 11-year follow-up study. J Arthroplasty 2012;27:533-8.e1. [Crossref] [PubMed]
- Gaillard-Campbell MD, Gross TP. Femoral fixation methods in hip resurfacing arthroplasty: an 11-year retrospective comparison of 4013 cases. J Arthroplasty 2019;34:2398-405. [Crossref] [PubMed]
- Glyn-Jones S, Pandit H, Kwon YM, et al. Risk factors for inflammatory pseudotumour formation following hip resurfacing. J Bone Joint Surg Br 2009;91:1566-74. [Crossref] [PubMed]
- De Haan R, Pattyn C, Gill HS, et al. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br 2008;90:1291-7. [Crossref] [PubMed]
- Williams S, Leslie I, Isaac G, et al. Tribology and wear of metal-on-metal hip prostheses: influence of cup angle and head position. J Bone Joint Surg Am 2008;90:111-7. [Crossref] [PubMed]
- Tiberi JV 3rd, Antoci V, Malchau H, et al. What is the fate of total hip arthroplasty (THA) acetabular component orientation when evaluated in the standing position? J Arthroplasty 2015;30:1555-60. [Crossref] [PubMed]
- Le Duff MJ, Ebramzadeh E, Amstutz HC. Contact patch to rim distance: the quintessential tool for metal-on-metal bearing in vivo performance analysis - a review. Hip Int 2017;27:220-5. [Crossref] [PubMed]
- Gross TP, Liu F. Outcomes after revision of metal-on-metal hip resurfacing arthroplasty. J Arthroplasty 2014;29:219-23. [Crossref] [PubMed]
- Hart AJ, Ilo K, Underwood R, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br 2011;93:315-20. [Crossref] [PubMed]
- Liu F, Gross TP. A safe zone for acetabular component position in metal-on-metal hip resurfacing arthroplasty: winner of the 2012 HAP PAUL award. J Arthroplasty 2013;28:1224-30. [Crossref] [PubMed]
- Langton DJ, Sprowson AP, Mahadeva D, et al. Cup anteversion in hip resurfacing: validation of EBRA and the presentation of a simple clinical grading system. J Arthroplasty 2010;25:607-13. [Crossref] [PubMed]
- Gaillard-Campbell MD, Gross TP. Prevention of metallosis in hip resurfacing: confirmation of the RAIL guideline in 2466 cases. EC Orthopaedics 2019;10:162-70.
- Hussey DK, McGrory BJ. Ten-year cross-sectional study of mechanically assisted crevice corrosion in 1352 consecutive patients with metal-on-polyethylene total hip arthroplasty. J Arthroplasty 2017;32:2546-51. [Crossref] [PubMed]
- Robinson E, Gaillard-Campbell D, Gross TP. Acetabular debonding: an investigation of porous coating delamination in hip resurfacing arthroplasty. Adv Orthop 2018;2018:5282167 [Crossref] [PubMed]
- Gross TP, Liu F. Prevalence of dysplasia as the source of worse outcome in young female patients after hip resurfacing arthroplasty. Int Orthop 2012;36:27-34. [Crossref] [PubMed]
- Gaillard MD, Gross TP. Reducing the failure rate of hip resurfacing in dysplasia patients: a retrospective analysis of 363 cases. BMC Musculoskelet Disord 2016;17:251. [Crossref] [PubMed]
- Haddad FS, Masri BA, Garbuz DS, et al. Primary total replacement of the dysplastic hip. Instr Course Lect 2000;49:23-39. [PubMed]
- Gaillard-Campbell DM, Gross TP. Optimizing acetabular component bone ingrowth: the wedge-fit bone preparation method. Adv Orthop 2019;2019:9315104 [Crossref] [PubMed]
- Gross TP, Liu F. Risk factor analysis for early femoral failure in metal-on-metal hip resurfacing arthroplasty: the effect of bone density and body mass index. J Orthop Surg Res 2012;7:1. [Crossref] [PubMed]
- Gross TP, Liu F. Reducing the risk of early femoral failure after metal-on-metal hip resurfacing arthroplasty. Eur Orthop Traumatol 2014;5:115-21. [Crossref]
- Gross TP, Liu F. Is there added risk in resurfacing a femoral head with cysts? J Orthop Surg Res 2011;6:55. [Crossref] [PubMed]
- Bisseling P, de Wit BW, Hol AM, et al. Similar incidence of periprosthetic fluid collections after ceramic-on-polyethylene total hip arthroplasties and metal-on-metal resurfacing arthroplasties: results of a screening metal artefact reduction sequence-MRI study. Bone Joint J 2015;97-B:1175-82. [Crossref] [PubMed]
- Wylde V, Hewlett S, Learmonth ID, et al. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain 2011;152:566-72. [Crossref] [PubMed]
- Anakwe RE, Jenkins PJ, Moran M. Predicting dissatisfaction after total hip arthroplasty: a study of 850 patients. J Arthroplasty 2011;26:209-13. [Crossref] [PubMed]
- Gross TP, Liu F. Metal-on-metal hip resurfacing with an uncemented femoral component. A seven-year follow-up study. J Bone Joint Surg Am 2008;90:32-7. [Crossref] [PubMed]
- Seagrave KG, Troelsen A, Malchau H, et al. Acetabular cup position and risk of dislocation in primary total hip arthroplasty. Acta Orthop 2017;88:10-7. [Crossref] [PubMed]
- Madanat R, Hussey DK, Donahue GS, et al. Early lessons from a worldwide, multicenter, followup study of the recalled articular surface replacement hip system. Clin Orthop Relat Res 2016;474:166-74. [Crossref] [PubMed]
Cite this article as: Gross TP. Hip resurfacing: is female gender an absolute or relative contraindication? Ann Joint 2021;6:23.


