
Reconstruction in orthopaedic oncology: frontier and horizon
Reconstruction of bony defect with satisfactory durability and function is an important issue for orthopaedic oncology. A huge progress has been achieved with emergence of many justified reconstruction methods (e.g., allograft, autograft, recycling tumor bone, rotationplasty, plating and nails, endoprostheses, spinal instruments) and endoprosthetic systems (e.g., KMFTR, GMRS, Stanmore, MUTARS) during the past four to five decades (1). Although abundant experiences have been gained in reconstruction for defects around the hip, shoulder, elbow, knee and acetabulum, optimization is still possible and demanded.
Where are we?
Proximal femur
Proximal femur is a common site for primary osteogenic sarcoma accounting for about 16% Ewing’s sarcoma, 13% chondrosarcoma and 10% osteosarcoma (2). Proximal femoral replacement (PFR) has been the most popular and mature method for reconstruction after resection of the proximal femur because satisfactory functional status and high longevity of the implant are guaranteed (2-4). Complications are relatively rare. It was reported that the probability of a patient surviving aseptic loosening for 10 years was 93.8% (5). Dislocation of the hip has been a concern for PFR but the risk was low ranging from 2.7% to 5.7% (3,4). Augmentation of the hip capsule and abductors with artificial ligaments can further improve the stability and function of the endoprosthesis (6). Bipolar hemiarthroplasty is recommended but groin pain and acetabular erosion might occur. However, the risk is low and only about 5% of the cases would need conversion to total hip replacement during follow-up (3,7).
Proximal humerus
There are multiple reconstruction methods after resection of the proximal humerus (8,9). The prosthetic methods included proximal humeral replacement (PHR), allograft-prosthetic composite (APC), and the reverse total shoulder arthroplasty (rTSA), while the non-prosthetic methods included osteoarticular allograft, arthrodesis with different kinds of autografts, and Clavicula Pro Humero (CPH). Generally, PHR is the most common method because it is convenient to handle and provides satisfactory cosmetic appearance as well as acceptable function of upper limb (majorly the function of elbow and hand) (9,10). However, the function of the shoulder joint depends greatly on the function of deltoid and subluxation of the prosthesis is a common complication (11-13). Arthrodesis could achieve permanent stability once the bone heals, and then the motion of the arm can be improved via the movement of the scapula (14-16). However, the surgical procedure of arthrodesis is complicated and mechanical complications are not rare (9).
Elbow
The elbow is an uncommon site for primary or metastatic bone tumors (17). Reconstruction after removal of a peri-elbow malignancy has been very difficult due to the high stress at the articulation and high demand for range of motion. Total elbow replacement, either with segmental prostheses or allograft-prosthesis composite, has remained as a mainstream for reconstruction (17-19). The functional outcomes were satisfactory with a mean Mayo Elbow Performance Score (MEPS) ranging from 75 to 82, a mean Musculoskeletal Tumor Society (MSTS) score raging from 73% to 84% (17-20). The rates of complications, however, were always very high (24–65%) compared with the prostheses of the shoulder, hip and knee (19). Neuropathy (5–27%), infection (4–23%), nonunion of allograft (9–83%) and aseptic loosening (16–30%) were common types of complications (17-20). Although the physical linking of the humeral and ulnar components could prevent subluxation or dislocation of the joint, it would bear high stress causing bushing wear at the hinge and would also transmit the stresses to the implant-cement-bone interface leading to osteolysis and finally loosening of the stem (19,21).
Knee
Endoprosthetic replacement has become the most popular method for reconstruction after resection of tumors around the knee for decades (1,4,22). With the evolution of the technology and experiences of clinical application, modifications on the designs have been made to improve efficacy and longevity of the prostheses, which included the development of modular implants, the application of different fixation methods (cemented or cementless), and the switch from a fixed hinge to a rotating hinge (5,23-29). It was summarized in a systematic review that the mean 5-, 10-, 15-, and 20-year implant survival of distal femoral replacement (DFR) were 78.3%, 70.1%, 61.6%, 38.3%, slightly higher than that of proximal tibial replacement (PTR) (75%, 60%, 55.3%, 25.1%). Aseptic loosening (8.8%) and infection (8.5%) were the most common major complications in DFR, while in PTR, infection (16.8%) was the most threatening complication (30). Moreover, there is no solid evidence supporting that cementless fixation has superiority over cemented fixation in preventing aseptic loosening, or vice versa. Rotating-hinge mechanism might improve long-term implant survival and reduce bushing wear, but not necessarily prevented aseptic loosening compared with fixed-hinge mechanism (30). To develop a more durable knee endoprosthesis, efforts should be focused on reducing loosening and infection.
Pelvis
Limb-salvage surgeries for pelvic tumors have always been challenging because it requires removal of the tumor with a satisfactory margin while preserving a limb that would exert better function than amputation. The principles for reconstruction of pelvic defect include restoration of normal loading transfer and restoration of a functional hip. Reconstruction after type I/I+IV resection is not mandatory but recommended according to the literatures, because an unreconstructed iliosacral defect can result in Trendelenburg gait, proximal and medial migration of the acetabulum, leg length discrepancy and compensatory scoliosis during long-term follow-up (31-35). Common reconstructive methods include iliosacral arthrodesis with autografts or allografts, instrumental fixation such as screw-rod system, or combined (31-42). A peri-acetabular resection would surely demand for an active reconstruction to regain a functional hip with adequate stability (43). Biological methods such as arthrodesis, pseudoarthrosis, and hip transposition, have been reported to yield good function but are less used currently because of the prolonged immobilization, discrepancy of lower limbs and limited hip joint movement (32,42,44,45). Re-implantation of devitalized tumor bone with total hip replacement is also a feasible method but could not be used in case of massive bone destruction (46). Endoprosthetic reconstruction is a prior choice nowadays after decades of evolution through saddle prostheses, custom-made hemipelvic prostheses, ice-cream cone/pedestal cup prostheses and modular hemipelvic prostheses (47-51).
What can we learn from the past? The MAFOS model
Looking back at the past, we can summarize that five elements are required for a successful reconstruction method, which include Materials, Articulation, Fixation, Osseointegration, Soft-tissue reconstruction (we call it the “MAFOS” model).
Materials
Allograft, tumor-free autograft, recycling tumor bones and their combinations are common choice for biological reconstruction (52-54). Vitallium (CoCrMo alloy) and Titanium alloy have been the most popular materials for endoprostheses for several decades exhibiting good strength, durability and biocompatibility (1). In recent years, silver coating of the endoprosthesis has been applied and showed efficacy in reducing the risk of infection in some retrospective studies (55,56). Porous tantalum stocks have been also used instead of structural bone grafts for bone defects for non-neoplastic and neoplastic diseases with good midterm and long-term results (57-59). Carbon-fibre and PEEK might benefit oncological cases in terms of post-operative radiological evaluation but still need further clinical investigations to justify its efficacy and safety (60,61).
Articulation
Articulation is the key element of a megaprosthesis, which should provide adequate stability and range of motion. To fulfill this goal, the mechanical principles of human joints were extracted and translated into the designs of the prostheses. The mechanism for stabilization, the dimensions of motion, the impetus for movement, and the distribution and transmission of the stress, are all fundamental factors for considerations. Stability is the priority of an articulation, which could be fulfill by hardware machinery, hinge or locking mechanism, and ligamentous augmentation. Dimensions of motion sometimes would be reduced purposely in prosthetic design to obtain enough stability, such as the knee and elbow megaprostheses. In other cases, the reduction of dimensions of motion is due to the insufficiency of impetus (muscle strength), such as the shoulder and total femoral megaprosthesis. However, the improved stability by hardware mechanisms, which violates the nature of the human joint to some extent, will undoubtedly generate extra stresses in the articulation and fixation interface that finally causes bushing wear or loosening (e.g., knee and elbow megaprostheses).
Fixation
Fixation methods could be divided into extra-medullary and intramedullary fixation. Extra-medullary fixation generally means screws and plating, which is mostly used in biological reconstructions. Prosthetic design favors intramedullary fixation. Cementation and pressing-fit have been the two major methods for fixation of the intramedullary stem of megaprostheses for many years with relatively good outcomes (5,26,62,63). However, aseptic loosening/fixation failure remains as a constant risk increasing with time (30). Efficacy of the intramedullary fixation does not merely rely on the method itself, but also on the surgical technique of the surgeons, the matching between canal and stem, and the shearing stress on the stem. Augmentation with hydroxyapatite collar that inducing extra-cortical bridge might help decreasing risk of aseptic loosening (26). The fixation method of the Compress® prostheses is an innovation, which is secured with a stacked set of Belleville washers that functions as a spring producing an compressive force at the bone-prosthesis interface (64,65). However, new types of failures also occur making this new type of prosthesis fail to show obvious advantages over the previous ones (30,66).
Osseointegration
Osseointegration is probably the only way to achieve permanent fixation for reconstruction. Methods to achieve osseointegration include bone grafting and fixation, coating of intramedullary stem, and more recently tantalum and 3D-printed trabecular implants (43,52,57,63). 3D-printing technology could produce implants in any shape with a porous interface when needed. This attribute helps us to accomplish reconstruction for complicated bone defects.
Soft-tissue reconstruction
A proper reconstruction of soft-tissue attachments is of great benefit for protection, stabilization and mobilization of the megaprosthesis. Adequate coverage of the implants with soft tissue is essential for prevention of infection and extrusion of the implants, which depends on the volume of residual muscle and fascia, and the size and contour of the implants. Sometimes a flap would be needed for safety. The reason why we oppose a contour reconstruction with metal prosthesis for bone defects is the difficulties for soft-tissue coverage. This is very important when designing prosthesis for pelvic defect as someone might want to precisely reconstruct the contour of the ilium wing, which is actually of little use but of great threat of soft tissue complications. Repair of the joint capsule and peri-capsular muscles are important for stabilization and mobilization of the megaprosthesis, which sometimes needs artificial ligament for augmentation (6,10). A little trick for soft-tissue reconstruction is to design some holes in the prosthesis for suturing.
Where to go?
Under the instruction of the MAFOS model, we have made some attempts on changing current designs of some common endoprostheses.
Innovation of articulation
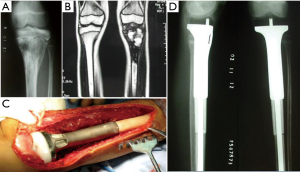
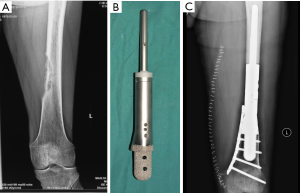
For children with proximal tibial defects, we found that hemiarthroplasty with artificial ligament reconstructing collateral and cruciate ligaments could provide enough stability and excellent functional status (Figure 1).
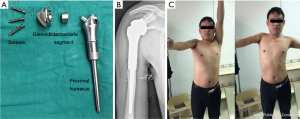
For defects of proximal humerus with sacrificing the deltoid and axillary nerve, we have designed and applied a 3D-printed arthrodesis-type megaprosthesis (Figure 2). The megaprosthesis consists of three components including the glenoid component, the intermediate segment, and the humeral component. The contour of outer interface of the glenoid component is designed to fit the shape of the articular surface of scapular glenoid. It is porous with proper pore size and porosity that facilitates bone ingrowth. Assembly of the three components could easily achieve shoulder arthrodesis. The functional outcomes were excellent with the mean MSTS-93 score as 25.4±2.1, the mean forward flexion of 78.0°±13.0° and abduction of 62.0°±11.5°, in a preliminary cohort of 9 patients.
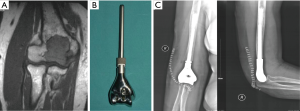
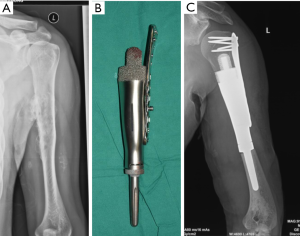
The advent of 3D-printing technology grants us more freedom for prosthesis design as it could produce implants in any shape. Straightforward duplication of the bone with metal is not feasible for many cases, but for the elbow joint, things may be different. We hypothesize that for the defect of distal humerus, hemiarthroplasty with a 3D-printed distal humeral endoprosthesis, which is designed according to the DICOM data of the contralateral humerus with mirror conversion, and reinforcement with artificial ligaments could provide enough stability and range of motion but less shearing stress on the intramedullary stem (Figure 3). Application of this method in a preliminary cohort of 5 patients has been resulted in satisfactory functional status and no major complications.
Refinement of fixation
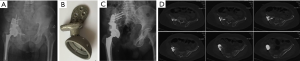
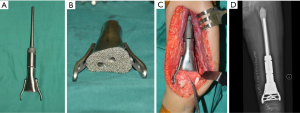
Again, new technology such as 3D-printing technology could refine the fixation of megaprostheses. For instance, we improved our previous modular hemipelvic prosthesis with 3D-printing technology, not only to fit the outer curved surface of the residual ilium more perfectly, but also to provide precise screw path from the implant through the sacroiliac joint into the body of S1 and S2 (Figure 4) (43). Fixation through the sacroiliac joint could transfer the weight loading like the normal pelvis and could avoid dissociation of the sacroiliac joint compared with the previous prosthesis.
Glamour of osseointegration
3D-printing technology could produce implants with a trabecular interface that could facilitate bone ingrowth. A retrieval study of a 3D-printed hemipelvic prosthesis in our center proved that new bone could grow into to the porous structure (67). Using the 3D printing, we have achieved several difficult reconstructions for intercalary defects that could not be reconstructed with commercial modular prostheses (Figures 5-7). Post-operative follow-up showed that all of them achieved satisfactory bone ingrowth. Despite the efficacy of 3D-printed trabeculae for osseointegration, it should be kept in mind that factors affecting bone union would definitely affect osseointegration into the prosthesis. A stable fixation, an interface as large as possible, a constantly compressive stress at the interface, and a proper porosity rate and pore size would undoubtedly benefit osseointegration.
The advent of new materials, new technology and new surgical techniques might bring in chances and new ideas to improve current methods for reconstruction in the field of orthopaedic oncology. However, we should remain sober and rational when developing new methods for reconstruction. Careful evaluation of the reconstruction plan and implant design with the “MAFOS” model is required before clinical application. Does the material have appropriate mechanical properties and osseointegration potential? Can we attain a stable and functional joint? Is the fixation durable enough? Is the soft-tissue reconstruction satisfactory? If all yes, then a successful reconstruction is guaranteed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Reconstruction in Orthopaedic Oncology - Frontier and Future Trends”. The article has undergone external peer review.
Conflict of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2020.01.07). The series “Reconstruction in Orthopaedic Oncology - Frontier and Future Trends” was commissioned by the editorial office without any funding or sponsorship. WG served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kotz RI. Progress in musculoskeletal oncology from 1922 - 2012. Int Orthop 2014;38:1113-22. [Crossref] [PubMed]
- Farid Y, Lin PP, Lewis VO, et al. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res 2006;223-9. [Crossref] [PubMed]
- Bernthal NM, Schwartz AJ, Oakes DA, et al. How long do endoprosthetic reconstructions for proximal femoral tumors last? Clin Orthop Relat Res 2010;468:2867-74. [Crossref] [PubMed]
- Henderson ER, Groundland JS, Pala E, et al. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am 2011;93:418-29. [Crossref] [PubMed]
- Unwin PS, Cannon SR, Grimer RJ, et al. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br 1996;78:5-13. [Crossref] [PubMed]
- Du Z, Tang S, Yang R, et al. Use of an Artificial Ligament Decreases Hip Dislocation and Improves Limb Function After Total Femoral Prosthetic Replacement Following Femoral Tumor Resection. J Arthroplasty 2018;33:1507-14. [Crossref] [PubMed]
- Houdek MT, Rose PS, Ferguson PC, et al. How Often Do Acetabular Erosions Occur After Bipolar Hip Endoprostheses in Patients With Malignant Tumors and Are Erosions Associated With Outcomes Scores? Clin Orthop Relat Res 2018; [Epub ahead of print]. [PubMed]
- Teunis T, Nota SP, Hornicek FJ, et al. Outcome after reconstruction of the proximal humerus for tumor resection: a systematic review. Clin Orthop Relat Res 2014;472:2245-53. [Crossref] [PubMed]
- Dubina A, Shiu B, Gilotra M, et al. What is the Optimal Reconstruction Option after the Resection of Proximal Humeral Tumors? A Systematic Review. Open Orthop J 2017;11:203-11. [Crossref] [PubMed]
- Tang X, Guo W, Yang R, et al. Synthetic mesh improves shoulder function after intraarticular resection and prosthetic replacement of proximal humerus. Clin Orthop Relat Res 2015;473:1464-71. [Crossref] [PubMed]
- Raiss P, Kinkel S, Sauter U, et al. Replacement of the proximal humerus with MUTARS tumor endoprostheses. Eur J Surg Oncol 2010;36:371-7. [Crossref] [PubMed]
- Fujibuchi T, Matsumoto S, Shimoji T, et al. New endoprosthesis suspension method with polypropylene monofilament knitted mesh after resection of bone tumors in proximal humerus. J Shoulder Elbow Surg 2015;24:882-8. [Crossref] [PubMed]
- Grosel TW, Plummer DR, Everhart JS, et al. Reverse total shoulder arthroplasty provides stability and better function than hemiarthroplasty following resection of proximal humerus tumors. J Shoulder Elbow Surg 2019;28:2147-52. [Crossref] [PubMed]
- Viehweger E, Gonzalez JF, Launay F, et al. Shoulder arthrodesis with vascularized fibular graft after tumor resection of the proximal humerus. Rev Chir Orthop Reparatrice Appar Mot 2005;91:523-9. [Crossref] [PubMed]
- Bilgin SS. Reconstruction of proximal humeral defects with shoulder arthrodesis using free vascularized fibular graft. J Bone Joint Surg Am 2012;94:e94 [Crossref] [PubMed]
- Barbier D, De Billy B, Gicquel P, et al. Is the Clavicula Pro Humero Technique of Value for Reconstruction After Resection of the Proximal Humerus in Children? Clin Orthop Relat Res 2017;475:2550-61. [Crossref] [PubMed]
- Weber KL, Lin PP, Yasko AW. Complex segmental elbow reconstruction after tumor resection. Clin Orthop Relat Res 2003;31-44. [Crossref] [PubMed]
- Athwal GS, Chin PY, Adams RA, et al. Coonrad-Morrey total elbow arthroplasty for tumours of the distal humerus and elbow. J Bone Joint Surg Br 2005;87:1369-74. [Crossref] [PubMed]
- Casadei R, De Paolis M, Drago G, et al. Total elbow arthroplasty for primary and metastatic tumor. Orthop Traumatol Surg Res 2016;102:459-65. [Crossref] [PubMed]
- Tang X, Guo W, Yang R, et al. Custom-made prosthesis replacement for reconstruction of elbow after tumor resection. J Shoulder Elbow Surg 2009;18:796-803. [Crossref] [PubMed]
- Sanchez-Sotelo J. Total elbow arthroplasty. Open Orthop J 2011;5:115-23. [Crossref] [PubMed]
- Allison DC, Carney SC, Ahlmann ER, et al. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012;2012:704872 [Crossref] [PubMed]
- Unwin PS, Cobb JP, Walker PS. Distal femoral arthroplasty using custom-made prostheses. The first 218 cases. J Arthroplasty 1993;8:259-68. [Crossref] [PubMed]
- Griffin AM, Parsons JA, Davis AM, et al. Uncemented tumor endoprostheses at the knee: root causes of failure. Clin Orthop Relat Res 2005;71-9. [Crossref] [PubMed]
- Myers GJ, Abudu AT, Carter SR, et al. The long-term results of endoprosthetic replacement of the proximal tibia for bone tumours. J Bone Joint Surg Br 2007;89:1632-7. [Crossref] [PubMed]
- Myers GJ, Abudu AT, Carter SR, et al. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br 2007;89:521-6. [Crossref] [PubMed]
- Schwartz AJ, Kabo JM, Eilber FC, et al. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop Relat Res 2010;468:2198-210. [Crossref] [PubMed]
- Mavrogenis AF, Pala E, Angelini A, et al. Proximal tibial resections and reconstructions: clinical outcome of 225 patients. J Surg Oncol 2013;107:335-42. [Crossref] [PubMed]
- Bus MP, van de Sande MA, Fiocco M, et al. What Are the Long-term Results of MUTARS® Modular Endoprostheses for Reconstruction of Tumor Resection of the Distal Femur and Proximal Tibia? Clin Orthop Relat Res 2017;475:708-18. [Crossref] [PubMed]
- Haijie L, Dasen L, Tao J, et al. Implant Survival and Complication Profiles of Endoprostheses for Treating Tumor Around the Knee in Adults: A Systematic Review of the Literature Over the Past 30 Years. J Arthroplasty 2018;33:1275-87.e3. [Crossref] [PubMed]
- Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am 1978;60:731-46. [Crossref] [PubMed]
- O'Connor MI, Sim FH. Salvage of the limb in the treatment of malignant pelvic tumors. J Bone Joint Surg Am 1989;71:481-94. [Crossref] [PubMed]
- Schwameis E, Dominkus M, Krepler P, et al. Reconstruction of the pelvis after tumor resection in children and adolescents. Clin Orthop Relat Res 2002;220-35. [Crossref] [PubMed]
- Aydinli U, Akesen B, Yalcinkaya U, et al. Iliosacral fixation after type-1 hemipelvectomy: a novel technique. Acta Orthop Belg 2012;78:393-7. [PubMed]
- Ogura K, Sakuraba M, Miyamoto S, et al. Pelvic ring reconstruction with a double-barreled free vascularized fibula graft after resection of malignant pelvic bone tumor. Arch Orthop Trauma Surg 2015;135:619-25. [Crossref] [PubMed]
- Kollender Y, Shabat S, Bickels J, et al. Internal hemipelvectomy for bone sarcomas in children and young adults: surgical considerations. Eur J Surg Oncol 2000;26:398-404. [Crossref] [PubMed]
- Beadel GP, McLaughlin CE, Aljassir F, et al. Iliosacral resection for primary bone tumors: is pelvic reconstruction necessary? Clin Orthop Relat Res 2005;22-9. [Crossref] [PubMed]
- Chang DW, Fortin AJ, Oates SD, et al. Reconstruction of the pelvic ring with vascularized double-strut fibular flap following internal hemipelvectomy. Plast Reconstr Surg 2008;121:1993-2000. [Crossref] [PubMed]
- Sabourin M, Biau D, Babinet A, et al. Surgical management of pelvic primary bone tumors involving the sacroiliac joint. Orthop Traumatol Surg Res 2009;95:284-92. [Crossref] [PubMed]
- Akiyama T, Clark JC, Miki Y, et al. The non-vascularised fibular graft: a simple and successful method of reconstruction of the pelvic ring after internal hemipelvectomy. J Bone Joint Surg Br 2010;92:999-1005. [Crossref] [PubMed]
- Nassif NA, Buchowski JM, Osterman K, et al. Surgical technique: Iliosacral reconstruction with minimal spinal instrumentation. Clin Orthop Relat Res 2013;471:947-55. [Crossref] [PubMed]
- Traub F, Andreou D, Niethard M, et al. Biological reconstruction following the resection of malignant bone tumors of the pelvis. Sarcoma 2013;2013:745360 [Crossref] [PubMed]
- Liang H, Ji T, Zhang Y, et al. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Joint J 2017;99-B:267-75. [Crossref] [PubMed]
- Gebert C, Wessling M, Hoffmann C, et al. Hip transposition as a limb salvage procedure following the resection of periacetabular tumors. J Surg Oncol 2011;103:269-75. [Crossref] [PubMed]
- Takami M, Ieguchi M, Aono M, et al. Flail hip joint following periacetabular tumor resection of the pelvis using upper surface of the femoral neck as a saddle: A case report. Oncol Lett 2015;10:3529-31. [Crossref] [PubMed]
- Chan LW, Imanishi J, Ngan SY, et al. Extracorporeal Irradiation and Reimplantation with Total Hip Arthroplasty for Periacetabular Pelvic Resections: A Review of 9 Cases. Sarcoma 2016;2016:2549616 [Crossref] [PubMed]
- Abudu A, Grimer RJ, Cannon SR, et al. Reconstruction of the hemipelvis after the excision of malignant tumours. Complications and functional outcome of prostheses. J Bone Joint Surg Br 1997;79:773-9. [Crossref] [PubMed]
- Ozaki T, Hoffmann C, Hillmann A, et al. Implantation of hemipelvic prosthesis after resection of sarcoma. Clin Orthop Relat Res 2002;197-205. [Crossref] [PubMed]
- Guo W, Li D, Tang X, et al. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin Orthop Relat Res 2007;180-8. [PubMed]
- Menendez LR, Ahlmann ER, Falkinstein Y, et al. Periacetabular reconstruction with a new endoprosthesis. Clin Orthop Relat Res 2009;467:2831-7. [Crossref] [PubMed]
- Ji T, Guo W, Yang RL, et al. Modular hemipelvic endoprosthesis reconstruction--experience in 100 patients with mid-term follow-up results. Eur J Surg Oncol 2013;39:53-60. [Crossref] [PubMed]
- Amin SN, Ebeid WA. Shoulder reconstruction after tumor resection by pedicled scapular crest graft. Clin Orthop Relat Res 2002;133-42. [Crossref] [PubMed]
- Innocenti M, Abed YY, Beltrami G, et al. Biological reconstruction after resection of bone tumors of the proximal tibia using allograft shell and intramedullary free vascularized fibular graft: long-term results. Microsurgery 2009;29:361-72. [Crossref] [PubMed]
- Liu T, Liu ZY, Zhang Q, et al. Hemicortical resection and reconstruction using pasteurised autograft for parosteal osteosarcoma of the distal femur. Bone Joint J 2013;95-B:1275-9. [Crossref] [PubMed]
- Hardes J, von Eiff C, Streitbuerger A, et al. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol 2010;101:389-95. [PubMed]
- Hardes J, Henrichs MP, Hauschild G, et al. Silver-Coated Megaprosthesis of the Proximal Tibia in Patients With Sarcoma. J Arthroplasty 2017;32:2208-13. [Crossref] [PubMed]
- Abdelaziz H, Jaramillo R, Gehrke T, et al. Clinical Survivorship of Aseptic Revision Total Knee Arthroplasty Using Hinged Knees and Tantalum Cones at Minimum 10-Year Follow-Up. J Arthroplasty 2019;34:3018-22. [Crossref] [PubMed]
- Houdek MT, Abdel MP, Perry KI, et al. Outcome of Patients Treated With Porous Tantalum Acetabular Implants for Neoplastic Periacetabular Lesions. J Am Acad Orthop Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Löchel J, Janz V, Hipfl C, et al. Reconstruction of acetabular defects with porous tantalum shells and augments in revision total hip arthroplasty at ten-year follow-up. Bone Joint J 2019;101-B:311-6. [Crossref] [PubMed]
- Miyazaki T, Matsunami C, Shirosaki Y. Bioactive carbon-PEEK composites prepared by chemical surface treatment. Mater Sci Eng C Mater Biol Appl 2017;70:71-5. [Crossref] [PubMed]
- Cawley DT, Alzakri A, Fujishiro T, et al. Carbon-fibre cage reconstruction in anterior cervical corpectomy for multilevel cervical spondylosis: mid-term outcomes. J Spine Surg 2019;5:251-8. [Crossref] [PubMed]
- Capanna R, Morris HG, Campanacci D, et al. Modular uncemented prosthetic reconstruction after resection of tumours of the distal femur. J Bone Joint Surg Br 1994;76:178-86. [Crossref] [PubMed]
- Pala E, Trovarelli G, Calabro T, et al. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clin Orthop Relat Res 2015;473:891-9. [Crossref] [PubMed]
- Farfalli GL, Boland PJ, Morris CD, et al. Early equivalence of uncemented press-fit and Compress femoral fixation. Clin Orthop Relat Res 2009;467:2792-9. [Crossref] [PubMed]
- Pedtke AC, Wustrack RL, Fang AS, et al. Aseptic failure: how does the Compress(®) implant compare to cemented stems? Clin Orthop Relat Res 2012;470:735-42. [Crossref] [PubMed]
- Healey JH, Morris CD, Athanasian EA, et al. Compress knee arthroplasty has 80% 10-year survivorship and novel forms of bone failure. Clin Orthop Relat Res 2013;471:774-83. [Crossref] [PubMed]
- Zhang Y, Tang X, Ji T, et al. Is a Modular Pedicle-hemipelvic Endoprosthesis Durable at Short Term in Patients Undergoing Enneking Type I + II Tumor Resections With or Without Sacroiliac Involvement? Clin Orthop Relat Res 2018;476:1751-61. [Crossref] [PubMed]
Cite this article as: Liang H, Guo W. Reconstruction in orthopaedic oncology: frontier and horizon. Ann Joint 2020;5:19.


