
Primary tumor resection in patients with metastatic osteosarcoma
Introduction
While osteosarcoma is a rare disease, it is the most common primary sarcoma of bone and has a predilection for affecting adolescents and young adults (1). Neoadjuvant, multi-agent chemotherapy has radically improved the survival of patients with non-metastatic osteosarcoma from approximately 20% in the 1970s to greater than 70% today (1-3). However, between 10 and 20% of patients with osteosarcoma present with metastatic disease and their survival rate remains extremely poor, with estimates between 11% and 19% (4-6).
Several studies have evaluated potential prognostic factors that influence survival, including tumor size, grade, patient age, and lymph node involvement (6-10). Of all the potential factors, the tumor stage at the time of diagnosis remains the most widely accepted and influential prognostic indicator (4,11). A review of 202 osteosarcoma patients with metastatic disease at the time of diagnosis further confirmed that the number of metastases at diagnosis and the completeness of surgical resection of all clinically detected tumor sites were also independent prognostic factors (11).
The decision to resect the primary tumor in the setting of metastatic disease remains highly controversial and needs to be tailored to each patient’s goals and their tumor characteristics. Recent studies demonstrated that patients who are able to undergo surgical resection of the primary tumor in the setting of metastatic breast cancer, colorectal carcinoma, and chondrosarcoma have an associated decreased mortality rate and prolonged survival time (12-15). However, the correlation between surgical resection and survivorship in patients with metastatic osteosarcoma remains unknown. Therefore, the purpose of this study was to evaluate the role of surgery in addition to demographic, socioeconomic, and tumor characteristics on the overall and cancer-specific mortality rate in patients with metastatic osteosarcoma.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) is a publicly available database that covers 34% of the U.S. population. The data is de-identified, case-based, and contributed by 20 geographically-defined cancer registries. These registries collect patient demographics, as well as data on the primary tumor anatomic location, morphology, disease stage at initial diagnosis, first course of treatment, and follow-up data. The population covered by all of these registries is comparable to the general U.S. population.
Study cohort
SEER was queried for all patients with metastatic osteosarcoma between the years 2004–2014. Inclusion criteria for the study were a histologic diagnosis of metastatic osteosarcoma as defined by an AJCC stage IV (derived from the 6th and 7th editions of the AJCC staging system) at the time of diagnosis. Patients were included if they underwent surgical resection of the primary tumor or if they were treated nonoperatively with adjuvant chemotherapy. Patients were excluded if the details of the surgery were unknown, if they were not treated with either surgery or chemotherapy, if they were diagnosed at autopsy, or if they did not have metastatic disease of a primary osteosarcoma.
Variables
Demographic data was also collected including age at the time of diagnosis, sex, race (Black, White, and other), and ethnicity (Hispanic or non-Hispanic). Insurance coverage (private or Medicare, Medicaid, uninsured, and unknown), marital status (married, single, divorced or widowed, and unknown), geographic population density at the site of patient residence (rural or urban location), and year of diagnosis were also queried.
Socioeconomic status (SES) was measured using a composite score calculation described in prior studies (16-18). SEER reports county-level data on household income, percent of the population living above the poverty line, percent of the population below the level of a high school education, and percent unemployment. Patients were grouped into quartiles for each of the four components (income, poverty, education, and employment) of the SES score, and patients in the lowest quartiles were compared to the rest of the population.
Tumor-specific variables included tumor size (in centimeters), grade (II, III, IV, and unknown), anatomic location, and histologic subtype as defined by the International Classification of Disease for Oncology (ICD-O-3). Histologic subtypes included conventional osteosarcoma as well as chondroblastic, fibroblastic, and telangiectatic. Patients were excluded if their histologic subtypes were surface (parosteal and periosteal) or secondary osteosarcomas (Paget’s and radiation-induced) as these subtypes confer significantly different prognoses than conventional subtypes. Anatomic locations were defined as axial, extremities, or other unusual locations such as the mandible, similar to previous studies (16). Treatment variables of interest included chemotherapy (yes or no/unknown) and the type of surgery (resection of the primary tumor vs. no cancer-directed surgery).
Statistical analysis
The primary outcomes of interest were risk factors for all-cause mortality and cancer-specific mortality. SEER reports disease-specific mortality from information abstracted from death certificates, which are subsequently reported as either “cancer” or “other causes”. Any death attributable to the primary tumor, disease recurrence, or sequela from metastatic disease is attributed to “cancer”. Secondary outcomes included 1, 3, and 5-year survival rates for patients with metastatic osteosarcoma. Survival time was defined as the number of months from diagnosis until death.
Frequencies of patient, tumor, and treatment characteristics were first calculated. Baseline covariates were analyzed using the Chi-square test or Student t-test for categorical or continuous variables, respectively. Simple, univariate Cox regression models were then created to assess the effect of each of the potential covariates on overall- and cancer-specific mortality. Variables included in the initial, unadjusted models included age, sex, race, ethnicity, insurance coverage, marital status, year of diagnosis, anatomic location, histology, tumor size, grade, chemotherapy, surgery, geographic location, education, income, poverty, employment, and the composite SES score. Multivariate Cox regression models were then constructed using only predictors with substantial measures of association (P<0.1). For both overall and cancer-specific mortality, these models included age, marital status, anatomic site, grade, chemotherapy, surgery, and education level. Kaplan-Meier survival curves were then constructed and 1, 3, and 5-year survival rates were abstracted from the survival curves. All statistical analyses were conducted using SPSS version 25, with two-sided statistical significance set a priori at P<0.05.
Missing data
Tumor size was missing for 104 patients (21.7%). All 479 patients were included in all analyses that did not account for this variable. When tumor size was accounted for in the univariate regression analyses, patients with missing data were excluded in those specific analyses. However, primary tumor size was not a significant predictor for either overall or cancer-specific mortality, and this variable was therefore excluded in the multivariate Cox regression analyses. Therefore, all 479 patients were included in the final, adjusted, multivariate models.
Results
Demographics
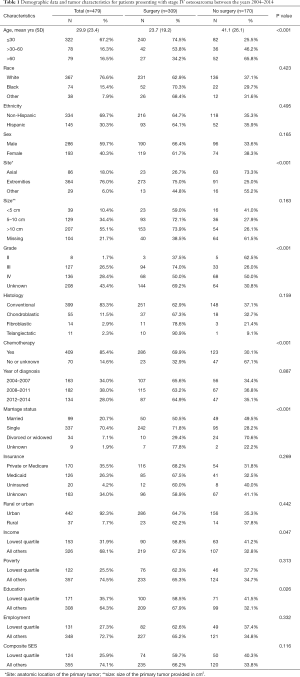
A total of 479 patients were identified for inclusion in this study (Table 1). Among them, 64.5% of the patients underwent surgical excision of the primary tumor while 35.5% were treated nonoperatively with adjuvant chemotherapy. Patients who were treated without surgery were significantly older and more likely to have an axial rather than an extremity-based primary tumor (P<0.001, Table 1). There were no significant differences based on race, ethnicity, sex, size of the primary tumor, or histologic subtype (P=0.159, Table 1).
Full table
No differences were observed with regards to geographic location of residence or composite SES scores and treatment arm (P=0.116, Table 1). Within the SES score, no differences were seen with employment or poverty levels (P=0.313, Table 1). However, patients in the lowest education quartile and the lowest income quartile were more likely to be treated nonoperatively (P=0.047, Table 1).
Overall mortality
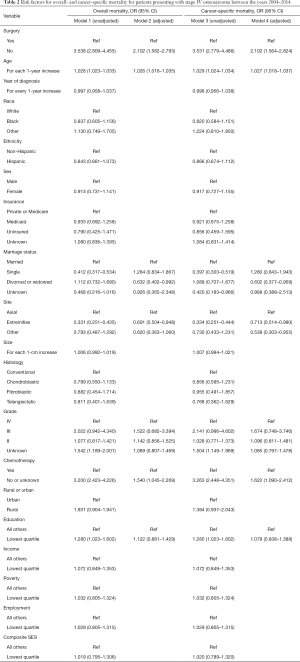
In the univariate model for overall mortality (Model 1 in Table 2), patients who did not undergo surgery had a significantly higher rate of overall mortality (OR 3.538, 95% CI: 2.809–4.455). The risk of all-cause mortality increased among older patients (2.8% per year, OR 1.028, 95% CI: 1.023–1.033) and those in the bottom quartile of education (OR 1.280, 95% CI: 1.023–1.602). Single patients, those who had received adjuvant chemotherapy, and those with an extremity location of their primary tumor had a decreased risk of all-cause mortality (P<0.001, Table 2).
Full table
The estimated ORs based on surgical treatment arm, age, anatomic location, and histology remained stable in the adjusted, multivariate model (Model 2 in Table 2). Marital status and education level lost statistical significance in the adjusted model. However, surgery remained the most predictive factor for overall survival (OR 2.102, 95% CI: 1.582–2.793).
Cancer-specific mortality
In the univariate analysis (Model 3 in Table 2), patients who did not undergo surgery had an increased risk of cancer-related mortality (OR 3.531, 95% CI: 2.779–4.486). The risk of cancer-related mortality increased with increasing patient age (OR 1.029, 95% CI: 1.024–1.034) and those in the bottom quartile of education (OR 1.280, CI 1.023–1.602). Patients who were single (OR 0.397, 95% CI: 0.303–0.519) as well as those who had received adjuvant chemotherapy (OR 3.263, 95% CI: 2.448–4.351), or had an extremity-based location of their tumor (OR 0.334, 95% CI: 0.251–0.444) had a decreased risk of cancer-specific mortality. Race, sex, ethnicity, insurance status, and year of diagnosis were not significant predictors for cancer-related mortality (Table 3). Furthermore, tumor size, histology, geographic location of residence, and SES score were also not significant predictors for the risk of cancer-specific mortality.
Full table
The estimated ORs based on surgical treatment arm, age, extremity location, tumor grade, tumor size, and adjuvant chemotherapy remained stable in the adjusted, multivariate model, while marital status and education level lost statistical significance (Model 4 in Table 3). However, surgery remained the most predictive factor for cancer-related survival (OR 2.102, 95% CI: 1.564–2.824).
Survival rates
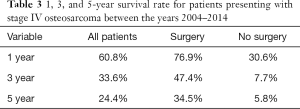
The mean 1-, 3-, and 5-year survival rates for patients with metastatic osteosarcoma were 60.8%, 33.6%, and 24.4%, respectively (Table 3). At all time points, a greater likelihood of survivorship was observed in patients who underwent surgical resection of their primary tumor (Table 3). At 5 years, patients who underwent surgery had a survival rate of 30.6% compared to 5.8% in patients treated nonoperatively.
Discussion
The results of this study demonstrate that 14.6% of patients with osteosarcoma present with metastatic disease at the time of diagnosis and their overall 5-year survival rate is 24.4%. Patients in the lowest quartile for income and education were more likely to be treated nonoperatively and surgical excision of the primary tumor was the strongest predictor of prolonged overall- and cancer-specific survivorship.
These findings mirror similar studies that report 11% of patients with osteosarcoma present with metastatic disease at the time of diagnosis and a 30.4% of these patients have a 5-year survival rate (5,11). In a single-center review of 202 patients who underwent surgery for metastatic osteosarcoma, Kager et al. found that poor histologic response to chemotherapy, more than one site of metastasis at the time of diagnosis, and incompleteness of surgical resection of all clinically detected tumor sites were most prognostic for mortality (11).
Prolonged survivorship associated with surgical resection of the primary tumor in the setting of metastatic disease has also been demonstrated in other populations including soft tissue sarcoma, chondrosarcoma, breast cancer, colorectal carcinoma, and renal cell carcinoma (19-21). Several possible explanations for these findings have been proposed. For one, removal of the primary tumor is thought to reduce the overall tumor burden as well as to remove the major source of cells that have gained metastatic competence (22). Additionally, the theory of ‘self-seeding’ postulates that circulating tumor cells from metastatic sites may return to the original site, promoting locoregional and systemic progression (22). While most tumor cells die within the hostile environment of the circulatory system, those that survive and return to the primary tumor via cytokines then contribute to a hospitable tumor microenvironment by suppressing immunosurveillance, promoting tumor growth and angiogenesis, and supporting further metastases (23,24). In fact, some animal models have demonstrated a reversal of immunosuppression following removal of the primary tumor, even in the presence of continued metastases (25).
However, literature on the removal of the primary tumor in the setting of metastatic disease remains highly controversial with mixed results. In other studies, uncontrolled growth of metastatic foci has been observed after resection of the primary tumor (26-28). The ‘dormancy hypothesis’ proposes that metastatic growth commonly includes periods of temporary dormancy at the single-cell phase and avascular micrometastasis phase. This theory suggests that surgery can drive escape from these dormant periods via the release of vascular endothelial growth factor (VEGF) and other unidentified proliferative inducers (29). This differs slightly from the theory of ‘concomitant tumor resistance’, which describes the ability of the primary tumor to slow metastatic growth through the secretion of antiangiogenic factors in the circulating system (27,30). Whether surgery drives escape from dormancy or removal of the primary tumor decreases antiangiogenic factors, both proposed pathways may consequently result in the uncontrolled growth of metastatic foci.
Surgery in the setting of metastatic osteosarcoma therefore remains a controversial decision and is predicated on many factors including the patient’s disease response to chemotherapy, pattern of metastatic disease, the palliation of symptoms, and the anatomic location and characteristics of the tumor itself (21). Ideally, prospective studies would shed further light on such issues, but the current topic does not lend itself to prospective studies. Retrospective studies such as this are limited by selection bias, whereby surgical excision may be preferentially offered to patients with a favorable perceived response to chemotherapy and a favorable pattern of metastases. Unfortunately, the authors are unable to comment on either of these potentially confounding characteristics due to the limitations of the SEER database. Furthermore, margin status post-surgery and the number of sites and location of metastatic disease were not available in the SEER database, and therefore remain limitations of these results. Additionally, patients with advanced metastases and poor overall health generally would not be considered a surgical candidate, further biasing retrospective results.
Conclusions
However, the present study demonstrates a strong association between primary tumor resection and survival in patients with metastatic osteosarcoma. Specifically, patients whose primary tumors and metastatic patterns are amenable to surgical resection have a much more favorable prognosis than those who are not able to undergo surgery. This information can be used to help identify patients with poor prognostic factors, educate and counsel patients and their families, and inform future studies to evaluate the role of surgery in metastatic osteosarcoma.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Kurt R. Weiss and Stella Lee) for the series “Osteosarcoma” published in Annals of Joint. The article has undergone external peer review.
Conflict of Interest: The series “Osteosarcoma” was commissioned by the editorial office without any funding or sponsorship. LRL reports KCI research support. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115:1531-43. [Crossref] [PubMed]
- Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol 2015;39:593-9. [Crossref] [PubMed]
- Jaffe N, Paed D, Farber S, et al. Favorable response of metastatic osteogenic sarcoma to pulse high-dose methotrexate with citrovorum rescue and radiation therapy. Cancer 1973;31:1367-73. [Crossref] [PubMed]
- Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20:776-90. [Crossref] [PubMed]
- Meyers PA, Heller G, Healey JH, et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol 1993;11:449-53. [Crossref] [PubMed]
- Kaste SC, Pratt CB, Cain AM, et al. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999;86:1602-8. [Crossref] [PubMed]
- Song K, Song J, Shi X, et al. Development and Validation of Nomograms Predicting Overall and Cancer-Specific Survival of Spinal Chondrosarcoma Patients. Spine 2018;43:E1281-9. [Crossref] [PubMed]
- Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome--the French pediatric experience. Cancer 2005;104:1100-9. [Crossref] [PubMed]
- Marina NM, Pratt CB, Rao BN, et al. Improved prognosis of children with osteosarcoma metastatic to the lung(s) at the time of diagnosis. Cancer 1992;70:2722-7. [Crossref] [PubMed]
- Ferguson WS, Harris MB, Goorin AM, et al. Presurgical window of carboplatin and surgery and multidrug chemotherapy for the treatment of newly diagnosed metastatic or unresectable osteosarcoma: Pediatric Oncology Group Trial. J Pediatr Hematol Oncol 2001;23:340-8. [Crossref] [PubMed]
- Kager L, Zoubek A, Pötschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 2003;21:2011-8. [Crossref] [PubMed]
- Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 2005;12:637-45. [Crossref] [PubMed]
- Clancy C, Burke JP, Barry M, et al. A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann Surg Oncol 2014;21:3900-8. [Crossref] [PubMed]
- Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery 2002;132:620-6; discussion 626-7. [Crossref] [PubMed]
- Song K, Song J, Chen F, et al. Does Resection of the Primary Tumor Improve Survival in Patients With Metastatic Chondrosarcoma? Clin Orthop Relat Res 2019;477:573-83. [Crossref] [PubMed]
- Miller BJ, Cram P, Lynch CF, et al. Risk factors for metastatic disease at presentation with osteosarcoma: an analysis of the SEER database. J Bone Joint Surg Am 2013;95:e89 [Crossref] [PubMed]
- Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950-2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health 2017;2017:2819372 [Crossref] [PubMed]
- Colton MD, Hawkins M, Goulding D, et al. Socioeconomics, Race, and Ethnicity in Childhood Cancer Survival: Accessing and Addressing Root Causes of Disparities. Cancer 2018;124:3975-8. [Crossref] [PubMed]
- Ramanathan RC, A'Hern R, Fisher C, et al. Modified staging system for extremity soft tissue sarcomas. Ann Surg Oncol 1999;6:57-69. [Crossref] [PubMed]
- Krishnan CK, Kim HS, Park JW, et al. Outcome After Surgery for Extremity Soft Tissue Sarcoma in Patients Presenting With Metastasis at Diagnosis. Am J Clin Oncol 2018;41:681-6. [Crossref] [PubMed]
- Ferguson PC, Deheshi BM, Chung P, et al. Soft tissue sarcoma presenting with metastatic disease: outcome with primary surgical resection. Cancer 2011;117:372-9. [Crossref] [PubMed]
- Criscitiello C, Giuliano M, Curigliano G, et al. Surgery of the primary tumor in de novo metastatic breast cancer: To do or not to do? Eur J Surg Oncol 2015;41:1288-92. [Crossref] [PubMed]
- Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell 2009;139:1315-26. [Crossref] [PubMed]
- Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016;529:298-306. [Crossref] [PubMed]
- Danna EA, Sinha P, Gilbert M, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res 2004;64:2205-11. [Crossref] [PubMed]
- Demicheli R, Retsky MW, Hrushesky WJM, et al. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: Learning from failures. Nat Clin Pract Oncol 2007;4:699-710. [Crossref] [PubMed]
- Tohme S, Simmons RL, Tsung A. Surgery for cancer: A trigger for metastases. Cancer Res 2017;77:1548-52. [Crossref] [PubMed]
- van der Bij GJ, Oosterling SJ, Beelen RHJ, et al. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg 2009;249:727-34. [Crossref] [PubMed]
- Retsky MW, Demicheli R, Hrushesky WJM, et al. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. Apmis 2008;116:730-41. [Crossref] [PubMed]
- Ruggiero RA, Bruzzo J, Chiarella P, et al. Concomitant tumor resistance: The role of tyrosine isomers in the mechanisms of metastases control. Cancer Res 2012;72:1043-50. [Crossref] [PubMed]
Cite this article as: Traven SA, Anderson AB, Walton ZJ, Leddy LR. Primary tumor resection in patients with metastatic osteosarcoma. Ann Joint 2019;4:46.


