Residual remnant preserving anatomic double-bundle anterior cruciate ligament reconstruction using hamstring tendon autografts
Introduction
Earlier studies reported that avascular necrosis occurred in the midsubstance of the tendon autograft after anterior cruciate ligament (ACL) reconstruction surgery (1). During the remodeling phase of the graft, the structural properties of the graft are decreased, and the reduced properties are not completely recovered even at 12 months after surgery (2). Recently, residual remnant tissue preservation of the ACL has attracted notice in ACL reconstruction (2-8). There is a strong possibility that residual remnant tissue preserving technique has several potential advantages to accelerate the graft remodeling process, such as improved knee stability, accelerated cell infiltration and revascularization, increased mechanoreceptors, and reduction of bone tunnel enlargement (6,9,10). Currently, the authors reported that the ligament remnant tissue preservation accelerated cell repopulation, revascularization, and regeneration of mechanoreceptors in the hamstring tendon autograft after ACL reconstruction using a sheep model (11). However, the effect of residual remnant tissue preserving technique on postoperative clinical outcome has not yet been fully understood in ACL reconstruction (6,12,13).
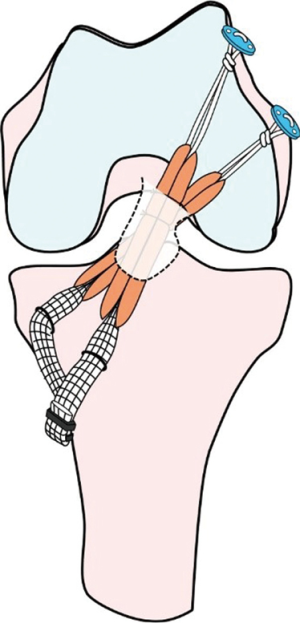
Several biomechanical studies reported that the double-bundle (DB) ACL reconstruction was significantly less in the postoperative side-to-side differences of knee laxity than the single-bundle (SB) reconstruction (14-16). However, clinical advantages of residual remnant tissue preservation in DB ACL reconstruction stay unclear. Recently, Yasuda et al. (10) have developed a novel procedure of remnant tissue preserved anatomic DB ACL reconstruction using the hamstring tendon hybrid autografts (Figures 1,2). Then, the authors compared clinical outcomes after anatomic DB ACL reconstruction between remnant tissue preservation procedure and remnant tissue resection procedure (17). In this article, the authors described the surgical technique and clinical outcomes of the residual remnant tissue preserving DB ACL reconstruction.

Surgical procedure
The patient is placed supine position on the operating table under general anesthesia. A pneumatic tourniquet is applied to the proximal thigh. Fluoroscopy comes in from the operative side of the operating table. The surgeon confirms to keep the femur horizontal, hanging the lower limb at 90° of knee flexion, and change the figure-4 position on the operation table. The patient undergoes draping with the lower limb placed in a stockinet to a position distal to the tibial tubercle under aseptic condition. Lactate Ringer solution is used for inflow with gravity.
A 3-cm oblique skin incision is made on the anteromedial (AM) aspect of the proximal tibia at 90° of knee flexion in the figure-4 position. After undermining the subcutaneous fat tissue, the sartorius fascia is exposed. The sartorius fascia is carefully elevated, and transversely cut. The semitendinosus tendon (Semi-T) and gracilis tendon (Gr) are undermined using the bent Kelly forceps. After the several fibrous bangs of the Semi-T are found and carefully cut, the Semi-T is harvested using an open-end tendon stripper. For graft preparation, the harvested Semi-T is cut in half. Each Semi-T is doubled using the circumferential ligation technique. A 10-mm mesh polyester tape (Leeds-Keio Artificial Ligament; Neoligaments, Leeds, England) is mechanically connected in series at a tibial part of each tendon (17) (Figure 2). When the doubled Semi-T is under 6 mm diameter or 24 cm length, the Gr is additionally harvested. After measurement of the femoral tunnel length, an EndoButton-CL-BTB (Smith and Nephew Endoscopy, Andover, MA, USA) is attached at the looped femoral part. Finally, colored 3-0 bioabsorbable sutures are attached to the grafts for flip markers. Tendon portions are inserted over 15 mm length to the tibial and femoral tunnels.
After injection physiological saline solution to knee joint cavity, standard anterolateral (AL) and AM parapatellar longitudinal portals are created. The ligamentum mucosum in the femoral notch and fat pad around the AL and AM portals are carefully removed by punch and shaver. Routine arthroscopy is performed to evaluate knee pathology, including meniscus and cartilage injuries.
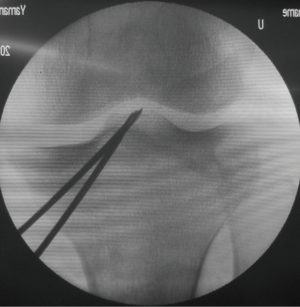
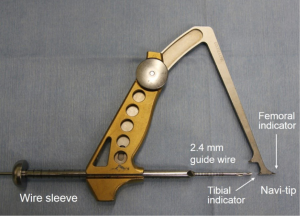
Before beginning the ACL reconstruction procedure, we arthroscopically saw the morphological status of the remnant tissue in each study participant. The knees with Crain type IV remnant tissue (18), the proximal end of which did not attach anywhere although the distal end attached on the tibia, were categorized as ‘the Non-remnant group’. The knees having Crain type I (remnant tissue attached the femoral notch), II [remnant tissue attached the posterior cruciate ligament (PCL)], or III (remnant tissue attached the femoral attachment of the ACL) remnant tissue, the proximal end of which attached on the femur or the PCL, were categorized as ‘the Remnant group’. Residual remnant preserving procedure is performed in patients who have an ACL remnant tissue of Type I, Type II, or Type III. The residual remnant ligament tissue preserved anatomic DB reconstruction procedure was previously reported in detail (10,17). The residual ACL remnant tissue is carefully saw using the probe. First, a guidewire for the tibial tunnel of the posterolateral bundle (PLB) is inserted using an originally developed hole-in-one guide (Wire-navigator, Smith and Nephew, Tokyo, Japan) which was developed for the transtibial tunnel technique (19,20) (Figure 3). This device consists of a tip part and a guidewire sleeve. The tip part has tibial and femoral indicators. The axis of the guidewire sleeve passes through the tip of the femoral and tibial indicators. The tip of the hole-in-one guide is placed at the center of the PLB attachment on the tibia from the lateral side of the tibial ACL remnant tissue (Figure 4A,B). The surgeon aims the femoral indicator at the center of the PLB attachment on the femur at 90° of knee flexion, keeping the femur horizontal (Figure 5A). The proximal end of the guidewire sleeve is fixed on the AM aspect of the tibia, and a guidewire is drilled through the sleeve into the tibia. The authors recommended that the tibial PLB tunnel angles averaged 45° measured as the angle subtended by the axis of the PLB tunnel and the long axis of the tibia in the anteroposterior view (21). To create the tibial AM bundle (AMB) tunnel, a longitudinal incision parallel to the remnant fiber orientation is made in the anterior aspect of the tibial ACL remnant tissue (Figure 5B). The tibial indicator is placed at the center of the AMB attachment on the tibia (Figure 4B). The femoral indicator is aimed at the center of the femoral AMB attachment (Figure 5A). Then, the surgeon should confirm whether the guidewire position is right using fluoroscopy for avoiding cartilage injury of the medial tibial condyle (Figure 6). Then, the tibial PLB and AMB tunnels are gently made with a cannulated drill. The surgeon should avoid injury of the tibial remnant tissue caught up by a drill.
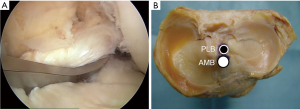
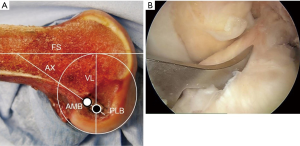
During surgery, the surgeon should keep the adherent femoral attachment of the ACL remnant tissue. A fibrous and synovial tissue around the femoral attachment of the ACL is carefully removed. A small incision is made parallel to the remnant fiber orientation on the femoral attachment of the remnant tissue at the 2 or 10 o’clock orientation by the knife. First, the dilator is gently introduced the tibial ACL remnant tissue through the tibial AMB tunnel. Then, a 5-mm offset guide (Transtibial Femoral ACL Drill Guide; Arthrex, Naples, FL) is inserted into the joint cavity through the tibial AMB tunnel (Figure 7A) at knee extension position. After the tip of off-set guide is placed on the posterior part of the femoral lateral condyle, a guidewire is inserted to the lateral femoral condyle at 100° of knee flexion. The femoral AMB tunnel is gently made with a 4.5-mm cannulated drill. After measurement of the femoral tunnel length using the stainless depth gauge, the femoral AMB tunnel socket is created using a half-cannulated drill (Smith and Nephew Endoscopy). The arthroscopic portal then is changed from the AL to the AM parapatellar portal. This is important to visualize the PLB attachment of the lateral femoral condyle. The center of the PLB attachment is marked by a bipolar device, keeping the femur horizontal. Then, the knee position is changed to the figure-4 position at 100° of flexion. A guidewire is inserted to the center of the femoral PLB attachment through the tibial PLB tunnel (Figure 7B) (20). The femoral PLB tunnel is gently made with a 4.5-mm cannulated drill. After measurement of the femoral PLB tunnel length using the stainless depth gauge, the femoral PLB tunnel is gently created using a half-cannulated drill, penetrating the remnant tissue (Figure 8A). During the tunnel creation, the bone debris should be removed using the shaver. The surgeon should avoid again injury of the femoral remnant tissue caught up by a drill.
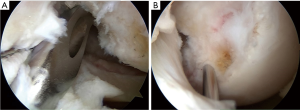
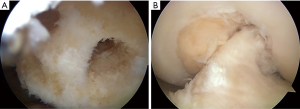
First, the PLB hybrid graft is introduced through the tibial PLB tunnel and the remnant tissue into the femoral PLB tunnel using the passing pin. An EndoButton is carefully flipped on the femoral cortical surface. Then, the AMB hybrid graft is fixed through the remnant tissue in the same manner (Figure 8B). The surgeon is confirmed that the 2 graft is covered the remnant tissue in the arthroscopic visual field. It should be confirmed that the remnant tissue and the grafts do not impinge on the femoral notch during the range of knee motion. The tensiometer (Yufu Itonaga Co., LTD. Tokyo, Japan) is attached to a polyester tape part of each graft. First, tension of 30 N to each graft is simultaneously applied at 90° of knee flexion, because the axis of the tibial tunnel is parallel to the axis of the femoral tunnel using the transtibial tunnel technique. Then, same tension is applied at 10° of knee flexion for 2 minutes using the tensiometers. The 2 tapes are simultaneously fixed onto the tibia using 2 staples (Smith and Nephew Endoscopy) (Figure 9). After irrigation to the tibial wound, layer suture is performed. Finally, 30° flexion soft knee brace is applied to the operated limb.
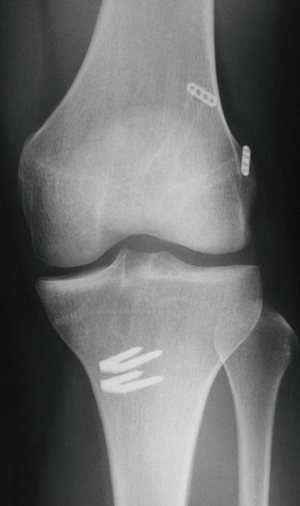
Pearls and pitfalls are shown in Tables 1,2; the surgeon confirms to keep the femur horizontal, hanging the lower limb at 90° of knee flexion for the identification of the anatomical femoral attachment of the ACL. The AM parapatellar portal is useful for identification of the femoral attachment of the AMB and PLB of the ACL. A Wire navigator device is a key instrument for the transtibial tunnel technique. The authors recommended that the tibial PLB tunnel angles averaged 45° using the transtibial tunnel technique. The surgeon should avoid injury of the remnant tissue caught up by a drill. The surgeon should keep the adherent femoral attachment of the ACL remnant tissue. Careful drainage and careful shaving of the bone debris reduce the risk of joint effusion. An intra-articular tibial cartilage injury is a complication with the transtibial tunnel technique. Fluoroscopic control prevents cartilage injury and tunnel malposition. The difficulty of the graft passage is a possibility. Careful probing and movement of the remnant tissue will avoid this complication.

Full table

Full table
Advantages and limitations are the following; Remnant preservation in ACL reconstruction may enhance cell repopulation and revascularization in the graft, resulting in acceleration of graft remodeling. Preservation of the normal tibial attachment of the ACL might result in better postoperative stability of the knee when compared with a remnant-resecting procedure. This is a technically demanding procedure—the surgeon considered risk of cyclops lesion after surgery.
Rehabilitation
Postoperative rehabilitation is performed according to our earlier studies (17,19,20). Quadriceps and hamstring muscle training are started at once after surgery with the knee brace. Simultaneous isometric contraction exercise of the quadriceps and the hamstrings is a safely useful method in early-stage after ACL reconstruction. The patella mobilization is also performed. The patients are confirmed their knee extension position without knee brace three times in a day. Immobilization of the knee is applied for 2 weeks after surgery. Range of knee motion exercise is started until 120° of knee flexion. The leg curl is started on prone position. The static squat exercise is started at 90° of knee flexion. Full weight-bearing is allowed using a hinged functional hard brace at 2 weeks after surgery. Various kinds of athletic training are performed after 4 weeks. No running is allowed until 4 months after surgery. Sports activity is allowed at 6 to 9 months.
Effects of remnant tissue preservation on clinical outcome
A total of 272 patients who had the unilateral ACL injury underwent anatomic DB ACL reconstruction using hamstring tendon hybrid grafts. One hundred thirty-three patients underwent remnant tissue preservation (the remnant group), and the remaining 139 patients underwent the remnant tissue resection (the non-remnant group). The demographic data of the two groups of remnant and non-remnant was shown in Table 3. The patients were evaluated 2 years after surgery. Results showed that no serious complications were experienced in either group. The side-to-side difference of anterior laxity measured with KT-2000 arthrometer at 30° of knee flexion was significantly less (P=0.004) in the remnant group than in the non-remnant group. The pivot-shift test was negative in 90.6% of the remnant group and 74.7% of the non-remnant group (P=0.004). The Lysholm score averaged 96.9 and 96.0 points in the remnant and non-remnant groups, respectively. The International Knee Documentation Committee evaluation showed that 98, 32, and 3 knees were graded as A, B, and C, respectively, in the remnant group, while 93, 41, and 5 knees were graded as A, B, and C, respectively, in the non-remnant group. In the 2nd look arthroscopic observations (22), the overall score in the remnant group were significantly better than in the non-remnant group (P=0.017). The cyclops lesion, which is defined synovial nodule >5 mm around the femoral notch without any clinical symptoms, was seen in15% of the cases and 17% in the remnant and non-remnant groups, respectively. There was no significant difference in the occurrence rate of the cyclops lesion between the two groups.

Full table
Discussion
In this paper, the surgical technique, and clinical outcomes of residual remnant tissue, preserving DB ACL reconstruction using hamstring tendon hybrid autografts were described. The postoperative anterior and rotational laxities were significantly better in the remnant tissue–preserving group than in the remnant tissue–resecting group. Concerning the 2nd look arthroscopic observation, the remnant tissue-preserving group was significantly better than the remnant-resecting group.
There were many variations in the percentage of identifiable residual ligament remnant tissue on primary ACL reconstruction. Crain et al. (18) reported that in 58% of the ACL reconstruction patients, identifiable ligament tissue was seen. Nakamae et al. (23) noted that 50% of patients had large ACL remnants bridging tibia and either femur or PCL. Maeda et al. (24) reported that there were no large ACL remnants in 4.8% of the patients. The authors also found that 45% of patients had a thick remnant tissue at time of primary ACL reconstruction.
The authors considered that why the postoperative side-to-side difference in the anterior knee laxity was significantly less by residual remnant preserving technique in anatomic DB ACL reconstruction. Several investigators noted that the graft revascularization is not saw in remnant tissue-resected ACL reconstruction procedure (1,25). Subsynovial and intra-fascicular vessels were found in the ACL remnant tissue (9). Recently, Wu et al. (26) reported that the blood flow of the ACL graft was measured with laser doppler system at 6, 12, 18, and 24 weeks after ACL reconstruction using a rabbit model. There were significant differences between the remnant preservation and remnant resection groups at 6 and 12 weeks after surgery. Also, they described that the CD34-positive vessels were significantly higher in the remnant preservation group than in the remnant resection group. More recently, the authors (11) examined the biological and biomechanical effects of residual ligament remnant tissue preservation on the hamstring tendon autograft in ACL reconstruction using a sheep model. The number of cells and blood vessels in the midsubstance of the graft were significantly greater in the remnant group than in the non-remnant group at 4 weeks after surgery. These results showed that the preservation of the residual remnant ligament tissue in ACL reconstruction accelerated the revascularization and repopulation in the ACL graft. Second, Sun et al. (27) reported that concerning the tendon-bone healing, a dense Sharpey’s fibers were seen in tibia bone tunnel after the remnant preserving ACL reconstruction using the rabbit model. The authors reported that the direct insertion structure which was the anatomical four layers structure was found in the tibial enthesis of the ACL remnant tissue at 12 weeks after remnant tissue preserving ACL reconstruction using a sheep model. Also, Masuda et al. (28) reported that bone tunnel enlargement after DB ACL reconstruction using computed tomography. Results showed that bone tunnel enlargement was significantly less in the remnant tissue preserving group than in the remnant tissue resecting group. Therefore, the broad attachment of the reconstructed ACL may contribute to the normal ACL function without severe bone tunnel enlargement (29,30). Thus, ACL attachment preservation might result in better postoperative knee laxity.
In conclusion, the anterior and rotational laxity stabilities were significantly better in the remnant tissue-preserving procedure than in the remnant tissue–resecting procedure without any detrimental effects.
Acknowledgments
Funding: This work was financially supported in part by a Grant-in-Aid for Scientific Research (16H03158) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Takeshi Muneta) for the series “Anatomic Reconstruction of Anterior Cruciate Ligament - Concept, Indication, and Its Efficacy” published in Annals of Joint. The article has undergone external peer review.
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2019.09.03). The series “Anatomic Reconstruction of Anterior Cruciate Ligament - Concept, Indication, and Its Efficacy” was commissioned by the editorial office without any funding or sponsorship. EK reports Grant/Research funding from Olympus Terumo Biomaterials, Japan, Smith and Nephew, Japan, Yufu Itonaga Co., Ltd., Japan, Mochida Pharmaceutical Co., Ltd., Japan, other from Centre for Sports Medicine, Hokkaido University Hospital. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jackson DW, Grood ES, Arnoczky SP, et al. Freeze dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am J Sports Med 1987;15:295-303. [Crossref] [PubMed]
- Kondo E, Yasuda K, Katsura T, et al. Biomechanical and histological evaluations of the doubled semitendinosus tendon autograft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med 2012;40:315-24. [Crossref] [PubMed]
- Adachi N, Ochi M, Uchio Y, et al. Anterior cruciate ligament augmentation under arthroscopy. A minimum 2-year follow-up in 40 patients. Arch Orthop Trauma Surg 2000;120:128-33. [Crossref] [PubMed]
- Ochi M, Adachi N, Deie M, et al. Anterior cruciate ligament augmentation procedure with a 1-incision technique: Anteromedial bundle or posterolateral bundle reconstruction. Arthroscopy 2006;22:463.e1-463.e5. [Crossref] [PubMed]
- Lee BI, Min KD, Choi HS, et al. Arthroscopic anterior cruciate ligament reconstruction with the tibial-remnant preserving technique using a hamstring graft. Arthroscopy 2006;22:340.e1-340.e7. [Crossref] [PubMed]
- Lee BI, Kwon SW, Kim JB, et al. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy 2008;24:560-8. [Crossref] [PubMed]
- Ahn JH, Lee YS, Ha HC. Anterior cruciate ligament reconstruction with preservation of remnant bundle using hamstring autograft: Technical note. Arch Orthop Trauma Surg 2009;129:1011-5. [Crossref] [PubMed]
- Löcherbach C, Zayni R, Chambat P, et al. Biologically enhanced ACL reconstruction. Orthop Traumatol Surg Res 2010;96:810-5. [Crossref] [PubMed]
- Dhillon MS, Bali K, Vasistha RK. Immunohistological evaluation of proprioceptive potential of the residual stump of injured anterior cruciate ligaments (ACL). Int Orthop 2010;34:737-41. [Crossref] [PubMed]
- Yasuda K, Kondo E, Kitamura N, et al. A pilot study of anatomic double-bundle anterior cruciate ligament reconstruction with ligament remnant tissue preservation. Arthroscopy 2012;28:343-53. [Crossref] [PubMed]
- Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med 2016;44:1708-16. [Crossref] [PubMed]
- Hong L, Li X, Zhang H, et al. Anterior cruciate ligament reconstruction with remnant preservation: a prospective, randomized controlled study. Am J Sports Med 2012;40:2747-55. [Crossref] [PubMed]
- Nakamae A, Ochi M, Deie M, et al. Clinical outcomes of second-look arthroscopic evaluation after anterior cruciate ligament augmentation: comparison with single- and double-bundle reconstruction. Bone Joint J 2014;96-B:1325-32. [Crossref] [PubMed]
- Kondo E, Merican AM, Yasuda K, et al. Biomechanical comparison of anatomic double-bundle, anatomic single-bundle, and nonanatomic single-bundle anterior cruciate ligament reconstructions. Am J Sports Med 2011;39:279-88. [Crossref] [PubMed]
- Kondo E, Merican AM, Yasuda K, et al. Biomechanical comparisons of knee stability after anterior cruciate ligament reconstruction between 2 clinically available transtibial procedures: anatomic double bundle versus single bundle. Am J Sports Med 2010;38:1349-58. [Crossref] [PubMed]
- Yagi M, Wong EK, Kanamori A, et al. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med 2002;30:660-6. [Crossref] [PubMed]
- Kondo E, Yasuda K, Onodera J, et al. Effects of remnant tissue preservation on clinical and arthroscopic results after anatomic double-bundle anterior cruciate ligament reconstruction. Am J Sports Med 2015;43:1882-92. [Crossref] [PubMed]
- Crain EH, Fithian DC, Paxton EW, et al. Variation in anterior cruciate ligament scar pattern: Does the scar pattern affect anterior laxity in anterior cruciate ligament– deficient knees? Arthroscopy 2005;21:19-24. [Crossref] [PubMed]
- Yasuda K, Kondo E, Ichiyama H, et al. Anatomic reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon grafts. Arthroscopy 2004;20:1015-25. [Crossref] [PubMed]
- Yasuda K, Kondo E, Ichiyama H, et al. Clinical evaluation of anatomic double-bundle anterior cruciate ligament reconstruction procedure using hamstring tendon grafts: comparisons among 3 different procedures. Arthroscopy 2006;22:240-51. [Crossref] [PubMed]
- Kondo E, Yasuda K, Ichiyama H, et al. Radiologic evaluation of femoral and tibial tunnels created with the transtibial tunnel technique for anatomic double-bundle anterior cruciate ligament reconstruction. Arthroscopy 2007;23:869-76. [Crossref] [PubMed]
- Kondo E, Yasuda K. Second-look arthroscopic evaluations of anatomic double-bundle anterior cruciate ligament reconstruction: relation with postoperative knee stability. Arthroscopy 2007;23:1198-209. [Crossref] [PubMed]
- Nakamae A, Ochi M, Deie M, et al. Biomechanical function of anterior cruciate ligament remnants: how long do they contribute to knee stability after injury in patients with complete tears? Arthroscopy. 2010;26:1577-85. [Crossref] [PubMed]
- Maeda S, Ishibashi Y, Tsuda E, et al. Intraoperative navigation evaluation of tibial translation after resection of anterior cruciate ligament remnants. Arthroscopy. 2011;27:1203-10. [Crossref] [PubMed]
- Howell SM, Knox KE, Farley TE, et al. Revascularization of a human anterior cruciate ligament graft during the first two years of implantation. Am J Sports Med 1995;23:42-9. [Crossref] [PubMed]
- Wu B, Zhao Z, Li S, et al. Preservation of remnant attachment improves graft healing in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy 2013;29:1362-71. [Crossref] [PubMed]
- Sun L, Zhou X, Wu B, Tian M. Inhibitory effect of synovial fluid on tendon-to-bone healing: an experimental study in rabbits. Arthroscopy 2012;28:1297-305. [Crossref] [PubMed]
- Masuda T, Kondo E, Onodera J, et al. Effects of remnant tissue preservation on tunnel enlargement after anatomic double-bundle anterior cruciate ligament reconstruction using the hamstring tendon. Orthop J Sports Med 2018;6:2325967118811293 [Crossref] [PubMed]
- Hara K, Mochizuki T, Sekiya I, et al. Anatomy of normal human anterior cruciate ligament attachments evaluated by divided small bundles. Am J Sports Med 2009;37:2386-91. [Crossref] [PubMed]
- Kawaguchi Y, Kondo E, Takeda R, et al. The role of fibers in the femoral attachment of the anterior cruciate ligament in resisting tibial displacement. Arthroscopy 2015;31:435-44. [Crossref] [PubMed]
Cite this article as: Kondo E, Masuda T, Shiota J, Iwasaki K, Onodera T, Yasuda K, Yagi T, Iwasaki N. Residual remnant preserving anatomic double-bundle anterior cruciate ligament reconstruction using hamstring tendon autografts. Ann Joint 2019;4:37.