Uncemented megaprosthesis stem fixation using “Scratch Fit” to achieve improved implant fixation
Introduction
Despite the success of oncologic megaprosthesis over the last the 30 years, the incidence of aseptic loosening and the associated clinical failures for cemented implants remain high (20–30%), as recorded in multiple long term clinical series (1-3). The most common oncologic implants involve distal femoral and proximal tibial resections where biomechanical rotational forces are high but implant biomechanics have had limited biomechanical investigations (4-6). The increased use of uncemented megaprosthesis implants has shown the possibility of better clinical results but also the need to have better instrumentation for the surgical implantation of uncemented implants. We are proposing a new distal femoral stem implantation technique to achieve more stable implantation and fixation with an uncemented megaprosthesis implant.
Methods
Specimens
Thirteen unpaired human cadaveric whole femoral specimens were sourced from LifeNet Health Northwest (Renton, WA) and handled according to University of Washington and CDC guidelines for biohazardous materials. The tissue was fresh-frozen and stored at −20 °C until preparation and testing. The first specimen was used for protocol development and omitted, while the remaining 12 specimens were included in the study.
Specimens were thawed in a water bath, and each femur dissected of all soft tissues and visually inspected for defects prior to test preparation. The distal end of each femur was subsequently resected via a 13 cm osteotomy, measured from the distal medial femoral condyle. Prior to stem insertion, the proximal end of each femur was prepared for biomechanical testing. A second osteotomy was carried out near the lesser trochanter before embedding (potting) the proximal end of the femoral specimen in bone cement (poly-methylmethacrylate). Two osseous screws were inserted bicortically and perpendicular to each other before potting to enhance fixation.
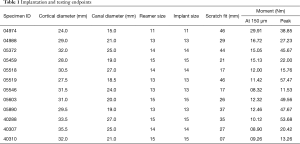
Femoral reaming and stem implantation was performed by a single orthopaedic surgeon (AWL) at the distal resection, osteotomy site (i.e., 13 cm from the distal end of the femur) (Figure 1). A short (125 mm) (straight fluted design from the Stryker GMRS uncemented press-fit system (Stryker Orthopaedics; Mahwah, New Jersey) was used, with stem sizes ranging from 11 to 15 mm (i.e., 11, 12, 13, 14, and 15 mm diameters). All stems were implanted via the usual Stryker technique and with a recommended vendor-supplied instrumentation set after measuring their respective scratch fit distances. Before reaming, cortical and intramedullary femoral diameters were measured in the anteroposterior (AP) and mediolateral (ML) planes. A short stem reamer, sized 0.5 mm smaller than the measured intramedullary diameters, was selected and reaming continued in a stepwise fashion (at 0.5 mm increments) until cortical chatter was obtained for most of the length of the femur. Each femoral specimen was under-reamed by 0.5 mm from the diameter of the chosen implant. A constant force was applied, using a 50 N (60 lbs.) spring release, custom insertion tool during the manual insertion of the stem into the distal femur. The distance between stem collar and femoral osteotomy site, defined as “scratch-fit”, was measured and recorded prior to completing the stem implantation (Figure 2, Table 1).

Full table
Experimental protocol
Torsional testing of the press fit femoral stem in the distal femoral specimen was carried out on a multi-axis biomechanical test frame, in conjunction with a Vicon 3-D motion analysis 4-camera system (Model MX13; Vicon Motion Systems; Lake Forest, CA) for the tracking of relative motion between implant and femur (Figure 3). Axial (torsional) moments were applied to the specimens at a controlled angular displacement rate (0.5 deg/sec) via one of three independently controlled rotary actuators (Model FHA-17C; HD Systems; Hauppauge, NY), allowing the femur to bend or displace in the other directions without constraint. A six-axis load cell (Omega 160; ATI Industrial Automation; Apex, NC) was used to record applied loads, sampled at a rate of 100 Hz by a connected data acquisition board (Model PCI 6034E; National Instruments; Austin, TX). Kinematics of both the implant and the distal end of the femur were captured using the Vicon system, which tracked attached reflective infrared targets at a 60 Hz sampling rate.
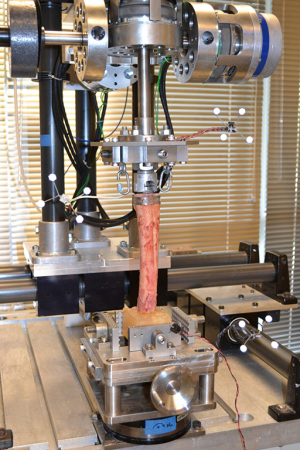
In order to simulate body weight, each specimen was preconditioned with a 700 N compressive preload during testing. A pneumatic cylinder, with Spectra fiber filament (Western Filament Inc.; Grand Junction, CO, USA) was attached to eyebolts that were fixed to the implant loading plate, employed for this purpose. The magnitude of the compressive load was adjusted by controlling the pressure of the cylinder so that that 700 N was introduced to the implant-bone construct at the beginning of each test. Off-axis loads caused by the compressive preload were minimized by using an X-Y stage to center each specimen on the load cell while securing in the multi-axis biomechanical test frame. After the compressive preload was engaged, a pure axial (torsional) moment was applied to each implant at a rate of 0.5 degrees per second. The test was stopped when the applied moment reached 50 Nm or failure (i.e., motion of the implant with respect to bone detected visually or via the load cell) was experienced. A custom designed LabVIEW virtual instrument (VI) (LabVIEW 7.0; National Instruments™; Austin, TX, USA), running on a Dell Precision Workstation 360 with an Intel Pentium 4 3.20 GHz processor with 512MB of RAM (Dell Computer Corporation; Round Rock, TX), was used for loading and data acquisition control. Load cell data was acquired with a high-speed multifunction data acquisition board (PCI-6220; National Instruments™; Austin, TX) with 16-bit resolution.
Data analysis
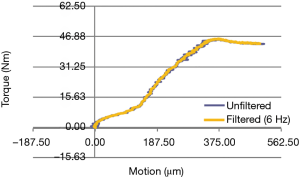
Standard Vicon analysis software packages (Vicon IQ and Body Builder; Vicon Systems; Los Angeles, CA, USA) were used to process acquired kinematic data, with the remaining data reduction and analysis done in Matlab (The Mathworks, Inc.; Natick, MA, USA) and R [R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria. http://www.R-project.org/.]. A sync light enabled synchronization of data sets, as the load cell data needed to be down sampled to 60 Hz in order to match the Vicon data set. Axial (torsional) moment versus relative angular displacement (between implant and distal femur) was the output of interest. Noise in the data was reduced using a Butterworth low-pass filter with a 6 Hz cutoff frequency and the relative angular displacement was subsequently converted to relative motion in µm.
Torsional stability was assessed using both the micromotion and failure endpoints. A micromotion endpoint was used because minimal movement is critical for achieving adequate uncemented stem fixation and excessive motion will result in fibrous rather than bony ingrowth. Torque was therefore evaluated for each specimen at the accepted micromotion threshold of 150 µm. Peak torque was also recorded as a proposed predictor of stem failure.
Results
Table 1 shows implantation and testing data. All femoral specimens were reamed line-to-line prior to stem insertion. The “scratch-fit” between stem collar and osteotomy site ranged from 7 to 46 mm with a mean value of 29.1±12.7 mm. Torque at the implant micromotion endpoint (150 µm) ranged from 8.3 to 29.9 Nm with a mean of 13.5±5.8 Nm, while peak torque had a mean of 33.6±17.0 Nm and ranged from 11.5 to 57.5 Nm.
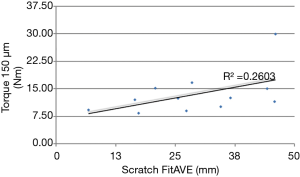
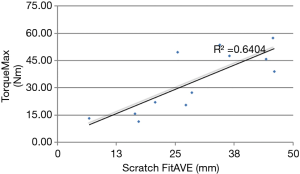
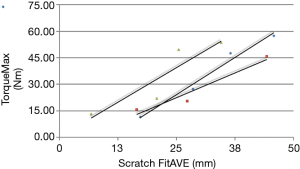
Biomechanical results are demonstrated in the following four figures. A axial-torsional moment versus micro-motion (150 micron motion between implant and distal femur) plot is shown in Figure 4. A Butterworth low-pass filter with 6 Hz cutoff frequency was used to smooth the curve. Figure 5 shows implant torque (Nm) to 150 micron (µm) of micro-motion vs. implantation “scratch fit” distance (mm). Figure 6 shows (Nm) peak implant torque versus implantation “scratch-fit” for all 12 specimens. As illustrated in Figure 5, there does not appear to be a correlation between “scratch-fit” and torsional moment at the micromotion endpoint (150 microns). However, Figure 6 exhibits a more significant relationship between peak torque (Nm) and implantation “scratch-fit” (mm) (r2 =0.64), a finding that is even more pronounced when evaluated also with stem diameter (mm) (Figure 7).
Discussion
Proposed factors for the determination of uncemented stem (implant) stability include factors associated with the femoral shaft (diameter, cortical strength, curvature), implant size, and the diameter of femoral reaming. The strongest apparent correlation in our testing groups was the comparison of peak torque to implantation “scratch fit” That correlation appears stronger when peak torque is correlated with implant diameter. Testing by peak torque vs. scratch fit for all implant sizes appeared to demonstrate a less significant correlation. The comparison of torque to initial micro-motion vs. scratch fit also appeared to be a less significant comparison. All biomechanical testing comparisons in the current experimental cohort appeared to have testing scratch fit (implantation) half way points at 2.5 to 3.0 cm’s suggesting that a minimal implantation “scratch fit” of 2.5 to 3.0 cm’s might serve as a preliminary threshold for uncemented stem stability or fixation. More detailed, future biomechanical testing with larger test cohorts and better controls for femoral reaming and femoral measurements will be required to achieve a better understanding of these biomechanical test groups.
Acknowledgments
The Authors would like to state their appreciation to Stryker Inc and LifeNet Health for the provision of Oncologic Implant Instrumentation and cadaveric specimens for this biomechanical study.
Funding: None
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Wei Guo, Tao Ji, Paul Jutte and Eric Henderson) for the series “Reconstruction in Orthopaedic Oncology - Frontier and Future Trends” published in Annals of Joint. The article has undergone external peer review.
Conflict of Interest: The series “Reconstruction in Orthopaedic Oncology - Frontier and Future Trends” was commissioned by the editorial office without any funding or sponsorship. Ernest U Conrad’s current relationship with LifeNet Health as a Medical Director, with minimal current reimbursement for those services ($3, 000 for research support). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The human cadaveric tissue utilized for this research was requested via a research tissue consent by the Northwest Tissue Center @LifeNet Health (Va Beach, Virginia), a tissue bank accredited by FDA review via Registration for Human Cells, Tissues and cell/tissue products, in addition to the American Association of Tissue Banks (AATB) and Certificates of Compliance for Clinical Laboratories (CLIA). Such programs are reviewed and approved by the FDA via corresponding federal legislation for human tissue and organs (NOPA 1984) and by corresponding Washington State legislation. The University of Washington does not routinely review requests regarding research utilizing human cadaveric tissue.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morgan HD, Cizik AM, Leopold SS, et al. Survival of Tumor Magaprostheses about the Knee. Clin Orthop Relat Res 2006;39-45. [Crossref] [PubMed]
- Henderson ER, Groundland JS, Pala E, et al. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am 2011;93:418-29. [Crossref] [PubMed]
- Capanna R, Scoccianti G, Campanacci DA, et al. What was the survival of megaprostheses in lower limb reconstructions after tumor resections? Clin Orthop Relat Res 2015;473:820-30. [Crossref] [PubMed]
- Taylor SJ, Walker PS. Forces and moments telemetered from two distal femoral replacements during various activities. J Biomech 2001;34:839-48. [Crossref] [PubMed]
- Viceconti M, Brusi G, Cristofolini L, et al. Primary stability of an anatomic cementless hip stem: a statistical analysis. J Biomech 2006;39:1169-79. [Crossref] [PubMed]
- Meneghini RM, Hallab NJ, Rosenberg AG, et al. Stem diameter and rotational stability in revision total hip arthroplasty: a biomechanical analysis. J Orthop Surg Res 2006;1:5. [Crossref] [PubMed]
Cite this article as: Conrad EU, Lindberg AW, White JK, Ching RP. Uncemented megaprosthesis stem fixation using “Scratch Fit” to achieve improved implant fixation. Ann Joint 2019;4:36.