Pelvic reconstruction using pedestal endoprosthesis—experience from Europe
Introduction
Primary tumours of the pelvis are rare, accounting for only 10% of all primary bone sarcomas. The bony pelvis is however, a common site for bony metastases (1). This high incidence, coupled with advances in oncological treatments resulting in increased survivorship of patients with metastatic disease (2), has seen the development of different reconstructive techniques following pelvic resection for oncological indications. Perhaps the greatest challenges offered for pelvic resection and reconstruction are following an Enneking and Dunham Type II and II/III resection, resections involving the acetabulum (3) due to the significant loss of bone stock and soft tissue constraints. At the extreme end of the surgical spectrum is the hindquarter amputation. However, this is associated with a high incidence of morbidity and mortality, as well as a significant psychological and physical burden placed by such a mutilating procedure, and therefore is often not the ideal choice for many patients (4).
Advances in imaging, oncological measures, and perioperative management have resulted in great advances in limb salvage surgery for periacetabular resections. The reconstruction of such defects has focused on improving the functional, psychological and cosmetic outcomes without compromising oncological outcomes. A multitude of prosthetic techniques have been described. Most recently the advent of 3D printing, and the evolution of custom prostheses has provided optimism that a better alternative for reconstruction in the limb salvage setting may have been found (5,6). However, the cost of implants and the high complication rate, does not always make this the best choice of reconstruction (7). Other reconstruction techniques include biological options such as allograft and extracorporeal irradiation and reimplantation, though these too are not without significant risk. Whilst a multitude of techniques have been described for periacetabular reconstruction, no consensus exists on whether any reconstruction confers a better function than no reconstruction at all. Indeed, significant geographical variation exists across the globe for how and if such defects are reconstructed (8). Surgeons in the UK had considerable experience in using custom made pelvic endoprostheses but these had high complication rates, especially infection.
The coned hemi-pelvic reconstruction prosthesis (Stanmore Implants, Elstree, UK) was first introduced in 2003 (Figure 1). The design ethos was to be simple to design and manufacture, easy to insert and to hopefully reduce the complications seen with other hemi-pelvic replacement prostheses. The design, and more importantly the surface coatings were aimed to provide fixation to even a minimal amount of residual ilium. The coned prosthesis was initially based on the Ring stemmed acetabular hip replacement prosthesis popular in the UK during the late 1960s and 1970s. The hemi-pelvic replacement is a pedestal endoprosthesis which allows fixation to the pelvis primarily via a conical fluted hydroxyapatite stem, press fit inside of the posterior column of the ilium. Rotational and axial stability is augmented with the addition of a block of antibiotic laden bone cement with the addition of strategically placed screws or threaded rods into the remaining ilium. An added benefit of the use of antibiotic laden cement was its use in preventing infection as there would be high levels of local antibiotic, much as in a first stage revision.
Following the initial successes of this design, other similar prostheses have since been introduced including the titanium pedestal cup (Zimmer-Biomet) and the LUMIC pedestal cup (Implantcast). Whilst these designs differ in their subtleties, the principles of reconstruction are similar across all prosthesis. Limited literature exists supporting the outcomes of pedestal cups. What does appear from the available literature is that such reconstructions are acceptable to patients and clinicians alike, and whilst the incidence of complications has been reduced with the use of such designs (for example, in comparison to the now outdated saddle-type prostheses), the risk of infection and instability remains high.
Available reports detailing the outcomes of such prostheses when applied to pelvic reconstruction date back to 2011. Fisher et al. (9) described the short- to medium-term outcomes of 27 patients implanted with the Stanmore hemi-pelvic replacement following pelvic sarcoma resection. The authors reported an overall survival of 70.4% at a mean of 39 months. For those who were still alive, the mean Toronto Extremity Salvage Score (TESS) was 69%. The incidence of dislocation was 14.8% and all dislocations occurred within the first 6 months following surgery. Deep infection occurred in 11.1% of patients, all of which occurred within the first 2 months of the operation.
Bus et al. (10) reported the mid- to long-term outcomes of the Zimmer titanium pedestal cup prosthesis in 19 patients with a mean follow-up of 39 months. The overall survival at final review was 63% with an implant survival of 50% at 5 years. The incidence of dislocation was 26% with an incidence of deep infection of 47%. Aseptic loosening requiring further intervention occurred in 16%. The mean Musculoskeletal Tumour Society (MSTS) Score for those who were able to contribute was 49%. Bus et al. (11) also reported on a series of acetabular reconstructions using the Implantcast LUMIC pedestal endoprosthesis following internal hemipelvectomy. Forty-seven patients were followed up for a minimum of 24 months. There was a mortality rate of 32%. Twenty seven patients (57%) had a complication of dislocation, infection, or loosening. The 5-year survivorship of the prosthesis was 83% and the mean MSTS at follow-up was 70%.
Hipfl et al. (12) reported the outcomes of 48 patients treated with a Zimmer titanium pedestal cup, including both primary and metastatic lesions. The authors reported a dislocation rate of 19%, an infection rate of 26% and an overall MSTS score of 74%, for those treated for a primary malignancy. Comparable results were seen in the metastatic bone disease group.
Barrientos-Ruiz et al. (13) reported on 10 hemipelvic resections reconstructed with a pedestal cup prosthesis (Stanmore). The authors reported a deep infection rate of 40%, though all patients were able to retain their prosthesis. No dislocations were reported in this small series. The overall MSTS score was 63%.
Issa et al. (14) published a series of 24 patients who underwent acetabular reconstruction for primary and metastatic pelvic disease as well as failures of previous reconstructions. There was an overall complication rate of 58% with infection (17%) and dislocation (17%) the two most prevalent complications. The implant needed to be removed in 9% of patients. The functional outcomes were good with mean MSTS of 72%.
De Paolis et al. (15) have also published similar results of acetabular reconstruction using an acetabular endoprosthesis in 45 patients. There was an overall complication rate of 40%, with a 10% dislocation rate and 13% deep infection. Eight percent of patients had their implant removed within the 5 years of follow-up. The mean MSTS at follow-up was 77%.
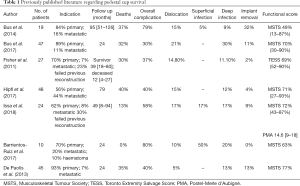
To summarise, therefore, such reconstructions appear rare in the available literature (Table 1). These series ranged from 10 to 48 patients with a follow-up period of 2–8 years. All papers reported on mortality and complication rates. They also reported a variety of functional scores including the MSTS score and TESS. The published overall complication rate ranges from 37% to 80% (6-10). The most common complication is infection in up to 20% of cases (13). Dislocation ranges from 4–17% while fracture of the ilium is around 20%. Functional scores were best in one of the largest series by De Paolis, who on average had a MSTS of 77% (including both primary and metastatic disease) (15). The lowest functional score was in a small series of 19 patients with a mean MSTS of 49% (10) (Table 1).
Full table
In 2011 we presented the first series of 27 patients who underwent acetabular reconstruction using the METS Coned hemipelvis for primary and metastatic pelvic malignancy (9). At this time the technique was novel and the series was small. There was an overall complication rate of 40%, which was predominantly dislocation (15%) and deep infection (11%). Since the publication of that initial series, our technique has been refined to address these high complication rates. These developments have included hypotensive epidural anaesthesia, the use of large diameter bipolar femoral heads, and the use of singular laterally based incisions. Parallel to these developments has been the use of intra-operative 3D navigation to facilitate both resection and reconstruction. This technology has been routinely used since 2009 and has altered the ability to accurately resect and reconstruct the pelvis in P2 and P2/3 resections (12). In an attempt to more accurately assess the medium to long-term outcomes and efficacy of this type of reconstruction, we have retrospectively analysed our experience with ice-cream cone type prostheses implanted in our institution for both primary and secondary malignancies of the pelvis, involving the P2 region
Methods
This preliminary, unpublished data relates to 52 patients undergoing acetabular reconstruction for primary or metastatic malignancy at the Royal Orthopaedic Hospital, Birmingham between 2004 and 2016. Outcomes recorded included survival and complications over the course of follow-up. Twenty-eight patients had a primary malignancy of bone while 24 were for metastatic disease.
All procedures were performed by specialist pelvic sarcoma surgeons. All patients were performed under combined spinal epidural regional anaesthesia with either sedation or general anaesthetic and prophylactic intravenous (IV) antibiotics. The type and dose of antibiotic varied over the 12 years of data collection, however, all patients received either a combination of 1 g of flucloxacillin with a renally titrated dose of gentamicin or 1 g of vancomycin with 1 g of meropenem. More recently, patients received tranexamic acid to minimise blood loss. The incisions utilised varied, determined primarily by the level of resection. These included a Kocher-Langenbach approach, Ollier’s lateral U, “reverse question mark” and an “S” shaped incision. The reconstruction requires the use of sequentially enlarging awls passed up the table of the ilium through the posterior column to create a tunnel of cancellous bone into which the stem can be inserted. The conical stem has a constant length of 93 mm with a pitch of 5 degrees but its diameter allows 4 options between 9 and 16 millimetres at its base. Whilst this impacted fluted, conical stem provides stability against axial compression, and a degree of rotational stability due to the fluted stem, further stability is afforded by screws placed both antegrade and retrograde between the prosthesis and the ilium, around which an antibiotic laden cement mantle is created. This cement enclave provides high doses of local antibiotics which elute from the cement, reduces the dead space, and increases the structural stability of the reconstruction. The inner diameter of the pedestal cup is 56 mm which allows the cementation of a 52 mm cup with a 2 mm cement mantle. In this series either a Trilogy Cup (Zimmer Biomet, Warsaw, Indiana) or SERF dual mobility cup (Société d’Etude, de Recherche et de Fabrication, Décines-Charpieu, France) were cemented into the pedestal endoprosthesis. Bearing options utilised included dual mobility, focal constraint, and large head metal on polyethylene bearing surfaces. The femoral component was reconstructed using either a cemented Exeter stem (Stryker, Michigan, USA) or a proximal femoral replacement (most commonly the METS system, Stanmore Implants Worldwide).
When the implant was first introduced, the placement of the implant was guided by the use of intra-operative fluoroscopy. As confidence in implant placement grew, this was replaced by an external marker to guide placement, an electrocardiography (ECG) dot on the posterior superior iliac spine which could be felt during surgery and towards which the stem of the prosthesis was directed. More recently, intra-operative navigation has been employed to enable the more accurate placement of the stem as well as facilitating the reproduction of appropriate cup anteversion, offset, and leg length.
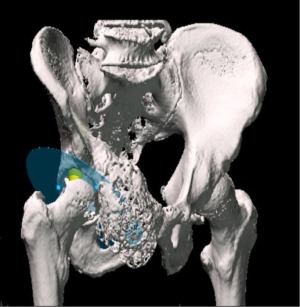
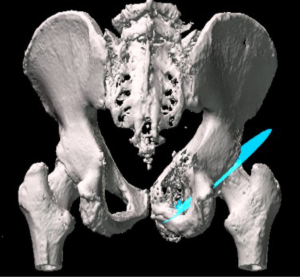
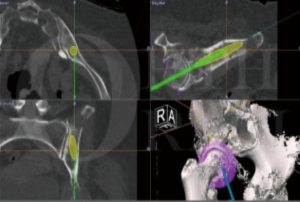
Patients who underwent navigated resection had a pre-operative computed tomography (CT) scan and magnetic resonance imaging (MRI), which was then fused together using the Stryker OrthoMap Navigation software (Stryker, Michigan, USA). From this, 3D high-resolution soft tissue and bony image resection margins were able to be created. This procedure has been previously published (16). Two techniques have been developed which allow intra-operative navigation to facilitate hip reconstruction using acetabular endoprosthesis. Firstly, a “ghost plane” is created which runs across the face of the acetabulum to establish acetabular version and inclination (Figures 2,3). This is important because once the resection has been completed there are no bony or soft tissue landmarks available to easily allow anatomical reconstruction. The “ghost plane” is then identified in space with the navigation pointer tool. The endoprosthesis can be inserted so that the face of the prosthesis overlies the “ghost plane”. Therefore, the hip centre of rotation, version and inclination of the acetabulum, and accurate offset can be accurately restored. The second technique is to navigate the pathway of the stem of the prosthesis into the posterior column towards the sacroiliac joint parallel to the sacroiliac bar. Pre-operative creation of a “screw” using the navigation software allows a template to guide placement of the stem of the prosthesis within the posterior ilium (Figure 4). This entry point and trajectory of this “screw” can be intra-operatively found using the navigation pointer and from this the surgeon can be sure that the stem of the prosthesis is being inserted in the correct position within the bone (Figure 4).
Post-operatively patients were treated with 5 days of prophylactic IV antibiotics and had compression stockings, sequential compression devices and chemical prophylaxis for 6 weeks for venous thromboembolic prophylaxis. Patients were mobilised following an X-ray showing a stable reconstruction. The subsequent follow-up was determined by the diagnosis; where primary sarcoma had follow-up according to current sarcoma guidelines, and those for metastatic disease were coordinated with the patients ongoing treatment.
Results
Between 2003 and 2016, 52 patients underwent acetabular reconstruction using a pedestal acetabular endoprosthesis for pelvic malignancy, 28 for a primary malignancy of bone and 24 for metastatic pelvic disease. Primary malignancies were chondrosarcoma (n=17), osteosarcoma (n=2), Ewing’s sarcoma (n=3), multiple myeloma (n=3), giant cell tumour (n=2), and lymphoma (n=1). The majority of metastatic disease was from breast carcinoma and renal cell carcinoma. There was an equal distribution according to gender. The average age at operation for all patients was 58 years (13–84 years), however as expected the mean age for patients being treated for primary bone malignancy was younger at 53 years (13–77 years) than for metastatic disease, which was higher at 64 years (41–84 years). There were 21 deaths (40%) with a mean time to death of 10 months. There were two peri-operative deaths (4%), one occurring intra-operatively and the other 2 weeks post-operatively from a cerebral infarct. The mean follow-up period was 36 months. Excluding patients who died, the mean follow-up was 54 months. Fourteen patients (27%) had a complication with 12 of these requiring further operations. Six patients (12%) suffered a dislocation; 4 patients’ dislocation was reduced with a closed reduction while 2 suffered recurrent dislocations which required revision of the bearing couple to a constrained prosthesis. Four patients (8%) had deep periprosthetic joint infections requiring washout and debridement. This was successful at eradicating infection in 2 (4%) whilst the remaining 2 (4%) required removal of the implant resulting in a hanging hip. One removal occurred at 4 months and one at 6 years post-operatively (Table 2). One patient who was successfully treated with debridement and IV antibiotics grew Staphylococcus lugdunensis which was sensitive to flucloxacillin. Similarly, another patient who had a sinus communicating with the prosthesis had negative cultures but was treated with 6 weeks of IV Tazocin followed by 6 weeks of oral flucloxacillin. The patients who failed debridement and required removal of their implant grew multiple organisms. One patient grew coliform, Peptococcus and Acinetobacter, while the other patient grew methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas.
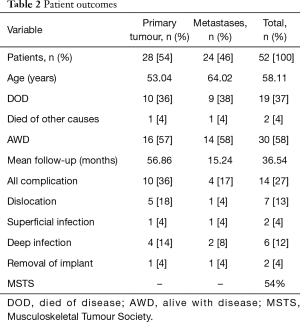
Full table
Functional outcomes for those who were alive at final follow-up were recorded using the MSTS score. The mean score was 54% with a range of 10–90%.
Navigation was used in 28 cases (54%). This group demonstrated a lower complication rate compared to the non-navigated group. In the navigated group there were 2 dislocations both of which reduced with a closed manipulation. Neither of these cases required further operations to increase constraint. In this group, infection occurred in 3 patients, 1 deep infection requiring an open debridement and 2 superficial infections (Table 3).
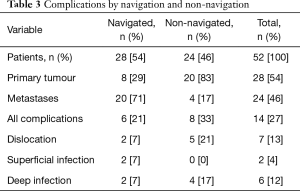
Full table
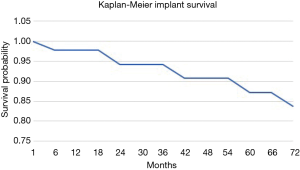
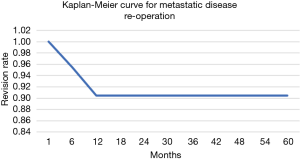
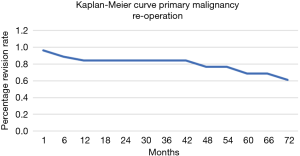
The overall survival of the prosthesis with revision of the pedestal cup for any cause was greater than 90% at 5 years (Figure 5). There were no revisions of implants in the group for metastatic disease, while the 5-year implant survivorship for the primary bone malignancy was 65%. When comparing the 5-year reoperation rate between the metastatic and primary bone malignancy group there was also a significant difference. The 5-year reoperation rate for metastatic disease was under 10% compared to 40% for primary bone malignancy (Figures 6,7). Whilst this is a crude measure of the implant survival, as given the patients for whom the implant was utilised, many will have succumbed to disease within a comparatively short period, it is reflective of the versatility of the reconstruction for these with primary malignancies of the pelvis, in whom long term patient survival is expected to be greater than 60% at 10 years.
Discussion
Pelvic resections for the treatment of either primary or secondary malignancy are associated with significant morbidity and mortality, a composite factor of the complexity of the surgery, anatomy, the difficulty of the reconstruction at restoring function, as well as the comorbidities often seen in this challenging patient group. The aim of reconstruction is to restore the centre of rotation of the hip, and leg length while providing a stable range of motion for the hip to perform functional tasks. In resections involving the acetabulum, the surgical complexity of reconstruction arises due to the loss of bony and soft tissue surrounding the hip joint, which ordinarily provide anatomical landmarks for reconstruction. Different reconstruction options exist, some of which have not survived the test of time, some of which remain to be proven. However, we have demonstrated through our series of ice cream cone acetabular endoprostheses, that this method of reconstruction is not only reliable but has provided a comparatively affordable, reproducible application with favourable outcomes, both functional and in terms of the incidence of complications.
As with all emerging surgical techniques, the techniques and applications evolve as experience grows. Following the first reports of its use, attempts have been made to reduce the incidence of complications. The introduction of hypotensive anaesthetic techniques, the use of an antibiotic laden cement void filler, and the use of navigation have resulted in a reduction in the overall complication rate, including the incidence of dislocation and infection. There has been a reduction of 10% in the overall complication rate to 27%, as well as a 3% reduction in the incidence of dislocation. Similarly, the deep infection rate has decreased by 3% since our first published series. Whilst the cause for this reduction is no doubt multifactorial, the reduction in dislocation is largely attributable to an expanded experience of the implant, the use of alternative bearing couples, and the use of 3D intra-operative navigation to recreate acetabular version, hip centre of rotation, and leg length using the “ghost plane” and navigated screw placement. The use of navigation, together with the use of a single laterally based incision, appear to have reduced the incidence of soft tissue complications and therefore infection rates. Similarly, the reduction in complications may also be attributed to the routine use of hypotensive epidural anaesthesia which has been shown in our series to lead to lower blood loss and rates of transfusion.
Twenty-one patients died with a mean follow-up of 9 months post reconstruction. Nineteen of these cases were due to disease progression, however two patients died in the perioperative period. One patient had a cardiovascular arrest following the cementation of the femoral component. Resuscitation was attempted during the operation but was unsuccessful. This patient was undergoing resection for metastatic renal cell carcinoma to the pelvis and proximal femur. The second patient underwent a P2 resection for grade II chondrosarcoma and died 2 weeks post-operative from cerebrovascular ischaemia.
Compared to other published data, these results show a similar distribution of complications, however the incidence of complications is below those of other published reports. Interestingly, the incidence of complications most similar to this was seen in a series published by Hipfl et al., which also had a similar size cohort. It is worthy of note that the series with the highest incidence of complications are those with the smallest numbers of patients. This is reflective of the steep learning curve of this challenging reconstruction technique.
There was a significant loss of follow-up due to mortality. Patients who had a pelvic resection for a primary bone sarcoma were followed up for a longer period, as one would expect. The mortality rate in this cohort was 50% so long-term follow-up has only been possible in 14 patients. It is in this population, and those who follow, from whom the long-term survival and late complications of this reconstruction technique will be learned.
Conclusions
Reconstruction of the periacetabulum as treatment for primary malignancies of bone, or due to their destruction by involvement by metastatic bone disease, remains one of the most challenging scenarios faced by orthopaedic oncological or pelvic reconstruction surgeons. The use of pedestal cups for such reconstructions have developed over time to be one of the most reliable methods of reconstruction. True to the design ethos, such implants have been shown to be simple to design and manufacture, easy to insert and with evolution, have reduced the complications seen with other hemi-pelvic replacement prostheses. This evolution has included not only included the use of navigated resection and reconstruction, but also advances in the perioperative management of patients undergoing pelvic resection, including the use of hypotensive epidural anaesthesia. The use of the acetabular endoprosthesis is a reliable reconstruction option which provides a stable hip joint for a greater period than the expected survivorship of the population in which it is used.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Wei Guo, Tao Ji, Paul Jutte and Eric Henderson) for the series “Reconstruction in Orthopaedic Oncology - Frontier and Future Trends” published in Annals of Joint. The article has undergone external peer review.
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2019.06.04). The series “Reconstruction in Orthopaedic Oncology - Frontier and Future Trends” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Royal Orthopaedic Hospital Institutional Review Board (No. 18-056). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Müller DA, Capanna R. The surgical treatment of pelvic bone metastases. Adv Orthop 2015;2015:525363 [Crossref] [PubMed]
- Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am 2000;31:515-28. vii. [Crossref] [PubMed]
- Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am 1978;60:731-46. [Crossref] [PubMed]
- van Houdt WJ, Griffin AM, Wunder JS, et al. Oncologic Outcome and Quality of Life After Hindquarter Amputation for Sarcoma: Is it Worth it? Ann Surg Oncol 2018;25:378-86. [Crossref] [PubMed]
- Holzapfel BM, Pilge H, Prodinger PM, et al. Customised osteotomy guides and endoprosthetic reconstruction for periacetabular tumours. Int Orthop 2014;38:1435-42. [PubMed]
- Perry KI, Abdel MP, Lewallen DG, et al. Innovative Methods of Reconstruction After Pelvic Tumor Resection. Curr Surg Rep 2014;2:41. [Crossref]
- Dai KR, Yan MN, Zhu ZA, et al. Computer-aided custom-made hemipelvic prosthesis used in extensive pelvic lesions. J Arthroplasty 2007;22:981-6. [Crossref] [PubMed]
- Gebert C, Gosheger G, Winkelmann W. Hip transposition as a universal surgical procedure for periacetabular tumors of the pelvis. J Surg Oncol 2009;99:169-72. [Crossref] [PubMed]
- Fisher NE, Patton JT, Grimer RJ, et al. Ice-cream cone reconstruction of the pelvis: a new type of pelvic replacement: early results. J Bone Joint Surg Br 2011;93:684-8. [Crossref] [PubMed]
- Bus MP, Boerhout EJ, Bramer JA, et al. Clinical outcome of pedestal cup endoprosthetic reconstruction after resection of a peri-acetabular tumour. Bone Joint J 2014;96-B:1706-12. [Crossref] [PubMed]
- Bus MP, Szafranski A, Sellevold S, et al. LUMiC® Endoprosthetic Reconstruction After Periacetabular Tumor Resection: Short-term Results. Clin Orthop Relat Res 2017;475:686-95. [Crossref] [PubMed]
- Hipfl C, Stihsen C, Puchner SE, et al. Pelvic reconstruction following resection of malignant bone tumours using a stemmed acetabular pedestal cup. Bone Joint J 2017;99-B:841-8. [Crossref] [PubMed]
- Barrientos-Ruiz I, Ortiz-Cruz EJ, Peleteiro-Pensado M. Reconstruction After Hemipelvectomy With the Ice-Cream Cone Prosthesis: What Are the Short-term Clinical Results? Clin Orthop Relat Res 2017;475:735-41. Erratum in: Erratum to: Reconstruction After Hemipelvectomy With the Ice-Cream Cone Prosthesis: What Are the Short-term Clinical Results? [Clin Orthop Relat Res 2017]. [Crossref] [PubMed]
- Issa SP, Biau D, Babinet A, et al. Pelvic reconstructions following peri-acetabular bone tumour resections using a cementless ice-cream cone prosthesis with dual mobility cup. Int Orthop 2018;42:1987-97. [Crossref] [PubMed]
- De Paolis M, Biazzo A, Romagnoli C, Alì N, Giannini S, Donati DM. The use of iliac stem prosthesis for acetabular defects following resections for periacetabular tumors. ScientificWorldJournal 2013;2013:717031 [Crossref] [PubMed]
- Morris GV, Stevenson JD, Evans S, et al. Navigation in Musculoskeletal Oncology: An Overview. Indian J Orthop 2018;52:22-30. [Crossref] [PubMed]
Cite this article as: Lowe M, Jeys L, Grimer R, Parry M. Pelvic reconstruction using pedestal endoprosthesis—experience from Europe. Ann Joint 2019;4:34.