
Pitfalls of revision reverse replacement part II: dealing with humeral bone loss and periprosthetic fractures
Introduction
Over the past decade, the utilization of reverse total shoulder arthroplasty (RSA) has increased in the United States (1). RSA was originally designed for patients with rotator cuff-deficient shoulders by inverting the ball-and-socket anatomy of the glenohumeral joint. The indications have grown to include glenohumeral arthritis (i.e., rheumatoid, osteoarthritis), acute and delayed treatment of complex proximal humerus fractures, failed shoulder arthroplasty, and tumors of the proximal humerus (1-4). Complications include instability, infection, scapular notching, acromion/scapular spine fractures, and periprosthetic fractures. Stability of the ball-and-socket is most dependent on proper tensioning of the soft tissues. Loss of proximal humerus bone stock can lead to insufficient lateral tensioning of the deltoid (5). In addition, it can be challenging to reattach the anterior and posterior deltoid, further contributing to instability. Furthermore, fixation of the humeral stem into only the diaphysis can be inadequate in patients with metaphyseal bone loss increasing the risk of loosening and periprosthetic fracture.
As the indications for RSA have expanded, complications related to the humerus, including significant humeral bone loss and periprosthetic fracture, have become more common. Proximal humeral bone loss can result from prior surgery, infection, or complex proximal humerus fractures in which the surgical neck or tuberosities progress to malunion, nonunion, or resorption. Additionally, after resection of an anatomic or reverse total shoulder, it is not uncommon for the proximal humerus, most commonly the greater tuberosity, to fracture and fail to subsequently heal. Periprosthetic RSA fractures occur in roughly 1% to 3% of cases, which can arise intra-operatively or postoperatively, which makes up close to 20% of all RSA complications (6-9). Risk factors include osteopenia, contracted soft tissues, and technical errors (7). Periprosthetic fractures can also result in deficiency of available humeral bone stock.
Classification of periprosthetic fractures
Wright and Cofield (6) in 1995 presented a classification system for humeral fractures after shoulder arthroplasty (Table 1). Type A fractures are located at the tip of the prosthesis and extend proximally, type B fractures lie at the tip of the prosthesis without proximal extension, and type C fractures are distal to the tip of the prosthesis. The authors recommended that type A fractures with a loose stem be treated with longer stem revision arthroplasty, and type A fractures with a well-fixed stem be treated with open reduction and internal fixation (ORIF). Type B and C fractures, which are long oblique or spiral, can be treated non-operatively if alignment is satisfactory and stable, whereas short oblique and transverse fracture should be treated with ORIF. These principles are largely still used today. There is a higher nonunion rate with type B fractures, so patients should be advised that a trial of non-operative management with these fractures may be unsuccessful (11). Andersen et al. (12) retrospectively reviewed a consecutive series of thirty-six patients with periprosthetic humerus fractures, 17 of which were around a reverse-geometry implant, and found low interobserver reliability (mean kappa, 0.37; range, 0.24 to 0.50) and a high intraobserver reliability (mean kappa, 0.69; range, 0.52 to 0.89) for the Wright and Cofield classification system. This makes it a useful but somewhat limited tool when evaluating patients.

Full table
Periprosthetic fractures can also be isolated to the greater or lesser tuberosity. In these cases, consideration should be given to fixation of the fractured tuberosity. Ohl et al. (13) demonstrated superior objective and subjective outcomes in RSA patients with greater tuberosity union when compared with patients with nonunion or excision. Autograft bone may be added to the tuberosity fixation and may improve union rates.
Measurement of humeral bone loss
Currently, there is no classification of humeral bone loss in the setting of shoulder arthroplasty, including RSA. However, preoperatively radiographs of the contralateral humerus can be obtained to compare to the operative side. Poltaretskyi et al. (14) proposed a novel, computerized model to calculate pre-morbid proximal humerus anatomy using 57 humeral CT scans with 3D humeral reconstructions in order calculate the 3D geometric parameters required to restore normal anatomy for patients undergoing shoulder arthroplasty. When including the metaphyseal region and mimicking osteoarthritis, the authors were able to prediction retroversion, inclination, height, radius of curvature and posterior and medial offset of the head of the humerus with errors of 2.9°±2.3°, 4.0°±3.3°, 1.0±0.8 mm, 0.8±0.6 mm, 0.7±0.5 mm and 1.0±0.7 mm, respectively. Future research is needed to determine the clinical application of the computer models and how they can help surgeons improve the function and outcomes of shoulder arthroplasty patients, especially those with RSA and humeral bone loss.
Postoperatively, humeral bone loss is measured on a standard anteroposterior radiograph. The measurement is the distance from the lateral aspect of the proximal end humeral prosthesis to the most proximal aspect of the remaining humeral bone (15). However, the actual magnitude of bone loss has been reported to be up to 1.5 cm greater than this measurement (15). A cut-off point that dictates management (no allograft vs. allograft vs. endoprosthesis) has not been determined.
Mechanism/prevention
Intraoperative iatrogenic fractures can occur most commonly during reaming of the humeral canal when there is excessive torque of the arm without allowing the arm to rotate or if resistance is met during reduction/dislocation maneuvers when longitudinal traction is not used; however, the usual lack of a rotator cuff makes iatrogenic fracture less common when compared with anatomic total shoulder arthroplasty. In osteopenic patients, a number of measures can be used to prevent intraoperative fracture. These include complete anterior and inferior capsular releases, use of a bone hook to deliver the humerus from the glenoid fossa and using a stem with a diameter smaller than the endosteal diameter of the diaphysis.
Management: periprosthetic fractures
Conservative
Intraoperative periprosthetic fractures are usually managed with immediate conversion to a longer stem or ORIF. It is generally advised to bypass the fracture by two cortical diameters (16). The management of postoperative fractures is more complicated; the treating surgeon must weigh a variety of factors to determine the need for surgical intervention. Non-operative management is indicated for patients unfit for surgery, certain non-displaced fractures with well-fixed stems (type B and C fractures), or patients refusing surgery. Activity modification, pain control, and frequent radiographic monitoring are the mainstays of treatment. Fracture braces may be beneficial depending on the nature and location of the fracture. The patient should be counseled that healing can take weeks to months, and there is a risk of non-union. Stiffness is also a common complication due to the prolonged immobilization for healing. A bone stimulator may be used for patients without evidence of union at three months. Wright and Cofield initially treated a patient after failed non-operative management with cerclage wire fixation, followed by bone grafting and electrical stimulation, which united at 33 months (6).
Revision
Surgical management of postoperative fractures is broadly divided into implant revision, where the humeral stem is removed and replaced, and implant sparing, where the stem is retained and a fracture is treated with extramedullary fixation. Implant revision is generally indicated for a loose prosthesis, whereas implant sparing techniques are generally used for well-fixed prostheses. When revising the implant, a stem that bypasses the fracture by at least two cortical diameters is recommended. Most reverse systems have a variety of humeral stem lengths in order to facilitate this. Longer revised stems provide a biomechanically superior construct to extramedullary fixation and are at lower risk for loosening; however, care should be taken to avoid intraoperative fractures, distal cortical perforation, and cement extrusion (17). In addition, removing the stem can be difficult and may worsen the fracture and/or comminution. The modularity of some shoulder arthroplasty systems can prevent this complication if portions are well-fixed. Sommacal et al. (18) reported a good outcome with a partial revision of a SMR reverse system with retention of the glenoid and humeral body and just conversion to a longer humeral stem.
Anderson et al. (12) presents a case series of reverse and anatomic periprosthetic humerus fractures divided by treatment group (ORIF vs. revision). Nine RSA patients were treated with revision arthroplasty; 7 of which had severe osteopenia. Time to union was 7.4 months (range 4 to 13.5). Three patients experienced complications including Morse taper dissociation, periprosthetic fracture initially treated non-operatively but became infected and required resection, and stem loosening treated conservatively.
Revision to a custom long-stem total shoulder replacement (TSR) is also an option for patients with humeral bone loss and/or component loosening. Sewell et al. (19) had satisfactory results in 4 patients with RSA periprosthetic fractures that were treated with custom TSR. Two fractures were type A, one was type B, and one was type C, and all four patients had severe osteopenia. No complications were noted.
ORIF
A variety of extramedullary fixation techniques may be employed when deciding to retain the implant. In the most basic sense, the stem can be ignored, and the fracture can be treated with a compression or locking plate (Figure 1). This can be difficult since the screws cannot be placed through the stem. In some instances, a cable can be used to augment the fixation of the plate. Another option is cortical strut allograft secured with cables. The strut allograft increases load dispersion of the cable and may incorporate into the humerus. Care should be taken when placing cables as not to incarcerate the radial nerve. Lastly, cables alone may be used in rare instances with non-displaced, long oblique fractures, but there is a high risk of cutting through the bone of the humerus. Overall, the main advantage of implant sparing is the decreased risk of damage with removing the prosthesis and worsening the fracture; however, biomechanically extramedullary fixation is usually weaker.
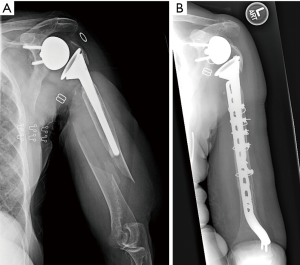
Martinez et al. (20) reported on 6 patients with type C periprosthetic fractures treated with broad 4.5 mm locking compression plate applied laterally through anterolateral approach. Bicortical screws were used for distal fixation, and cable wires for proximal and distal fixation. Strut allograft was applied medially and fixed with wires and distal bicortical screws. They achieved union of all fractures, and pre-facture shoulder range of motion and satisfaction attained in all but one patient at 12–17 months follow-up (20). Mineo et al. (8) described two cases of RSA patients with postoperative type C periprosthetic fractures, which were treated with locking plates with screws and cable wiring. Both fractures united by 5 months without complications. Andersen et al. (12) treated eight RSA patients with periprosthetic fractures with ORIF. Six of the eight had some degree of osteopenia. Time to union was on average 6.25 months (range, 4 to 12). Complications included baseplate and humeral socket fracture and distal fracture extension; both required revision surgery.
While choosing an implant revising versus an implant sparing management can be fairly straightforward based on the fracture characteristics, the implementation of the techniques is very much individualized and nuanced. Both strategies have proven to have satisfactory outcomes; however, patients should be advised that complications are not uncommon, and revision surgery may be indicated.
Management: humeral bone loss
The loss of humeral bone stock can affect component fixation as well as disruption of the insertion of the rotator cuff muscle (21). RSA is effective in treating failed shoulder arthroplasty because of its increased constraint and diminished reliance on the intact rotator cuff. Nevertheless, this implant relies on the humeral bone stock for implant fixation, rotational stability, and soft tissue attachment to improve function and stability. The loss of proximal humeral bone plays an important role in patient outcome; therefore, several options have been described to manage humeral bone loss during RSA, including the revision without allograft, allograft prosthesis composite (APC), and endoprosthetic replacement (Table 2).
Full table
Long stem component without graft
One option for treatment is revision of the humeral bone defect without the use of an allograft. Some surgeons recommend this technique due to significant concerns regarding the prosthetic allograft, including cost, increased risk of infection, donor to host reaction, increased operative time and complexity, graft resorption, and/or failure of graft incorporation. Surgeons favoring this technique report rotational and length stability of the prosthesis can be achieved with the use of long stem component, negating the need for allograft support. Also, that the semi-constrained nature of the reverse prosthesis and the ability to adjust the soft tissue tension with spacers results in a low rate of instability. Budge et al. (15) studied 15 patients with significant humeral bone loss (38.4 mm) who underwent RSA without allograft. Result showed an 87% satisfaction rate, a mean forward flexion of 103.2° and external rotation of 11.9°. Complications were notching in 3 patients, one anterior instability that required revision, and a periprosthetic fracture of one modular humeral stem. No subsidence or loosening was reported.
Shukla et al. (5) used a proximal humerus replacement system [Segmental Revision System (SRS); Zimmer-Biomet, Warsaw, IN, USA] to perform RSA on 34 patients. Indications included failed shoulder arthroplasty, tumor resection, malunion/nonunion, prior resection arthroplasty, and intraoperative fracture. Forward flexion improved from 31° to 109° (P<0.001), postoperatively. There was an 81% satisfaction rate. Eight patients (24%) required reoperations. The complications included humeral loosening (3 shoulders), periprosthetic fracture (2 shoulders), infection (2 shoulders), and dislocation (1 shoulder). An additional patient sustained a minimally displaced periprosthetic fracture successfully treated non-operatively.
APC
APC provides several theoretical benefits, including implants support, increase bone stock, restoration of humeral length, deltoid tensioning, an opportunity to repair the posterior aspect of the cuff to improve strength in external rotation and repair of the subscapularis to improved stability (Figure 2). It combines the durability of an endoprosthesis and the benefits of allograft (22,25).

Sanchez-Sotalo et al. (22) reviewed the results of 26 patients that received an APC, 8 primary and 18 revision. In this study, the fixation to hold the APC was performed with a compression plate. During a mean follow-up of 4 years, there were no significant differences in clinical outcomes between primary and revision case. No patients required revision surgery for nonunion at the host-allograft junction. The mean time to union was 7 months. The 2- to 5-year revision free survival rate was 96%.
Chacon et al. (23) evaluated the results of 25 patients with a reverse shoulder prosthesis allograft composite with a mean follow up was 30.2 months. The study shows improve score in the ASES and SST scores. The range of motion improved in flexion from 32.7° to 82.4° (P<0.001) and abduction from 40.4° to 81.4° (P<0.0001). And, 76% of patients reported a good or excellent result. Radiographic evaluation show incorporation of the allograft in the metadiaphysis in 84% of the patients and incorporation of the allograft in the diaphyseal region in 76% of the patients (23).
Martinez et al. (26) reported on 6 patients, who received a RSA and an APC for proximal humerus nonunion and extensive proximal bone loss. Two of the patients had postoperative infections, which required one or more surgeries and long-term antibiotics to treat. Two others had recurrent postoperative dislocations, which required revision surgery to a larger glenosphere. No further dislocations occurred thereafter. The remaining two patients who did not experience a complication were satisfied or very satisfied and would undergo the procedure again. APC is a viable option for humeral bone loss in the setting of RSA, but there is a high complication rate.
Endoprosthetic replacement
Endoprosthetic replacement was originally designed for reconstruction after limb salvage surgery. Almost no study has reviewed the results of these procedures for humeral bone loss management in a revision RSA. Nevertheless, it is an option for the management of humeral bone loss. Kumar et al. (11) performed a study in 47 patients that had a tumor reconstruction with endoprosthesis after limb salvage surgery, with a mean follow-up of 9 years. The mean length of the replaced humerus was 17 cm. The study showed survival of the prostheses of 86.5% at 20 years, and that the functional outcome was influenced by the size of the bone left after the resection. Cannon et al. (24) performed a study in 83 patients who underwent proximal humeral endoprosthetic reconstruction following intra-articular deltoid muscle and axial nerve sparing resection. Mean follow up was 30 months. Results showed active abduction of 41°, mean active forward elevation of 42°. Complications included 2 deep infections. No prosthesis was loosened.
Management of humeral bone defect can be challenging. Further studies reviewing the results of the multiple options in revision RSA will give us a better understanding of their role in this condition. Management for humeral bone defect will be dictated in great part by the amount of bone loss. Prosthetic revision without allograft showed good results for small to medium size defects. Allograft prosthetic composites may be the better option for medium to large defects of the humerus, based on the ability to reconstitute the bone stock, reattach the subscapularis tendon insertion, lateralizing the pull of the deltoid, and improved the contour of the shoulder. Various reconstruction techniques for the proximal humerus lead to relatively similar functional results. Surgical choice should be tailored to anatomic defect and functional requirements.
Conclusions
Fortunately, periprosthetic fractures are a rare, but potentially devastating complication of RSA. Non-operative management is possible for certain fractures with good alignment and a stable implant. Operative management whether implant revising or implant sparing is very much individualized for the type of RSA implant and nature and location of the fracture. Either operative strategy has a relatively high complication rate, and non-operative management frequently leads to non- or malunion. In addition, humeral bone loss in the setting of RSA is a complex problem with several potential treatment options that also have high complication rates.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Joseph A. Abboud) for the series “Evolving Trends in Reverse Shoulder Arthroplasty” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.11.12). The series “Evolving Trends in Reverse Shoulder Arthroplasty” was commissioned by the editorial office without any funding or sponsorship. AG reports personal fees from Wright Medical, personal fees from DJO, other from Smith and Nephew, other from Arthrex, personal fees from Journal of Bone and Joint Surgery, during the conduct of the study; and Chair of American Shoulder and Elbow Surgeons Shoulder Prosthetic Joint Infection Task Force and Section Organizer International Consensus Meeting on PJI- Shoulder. ESP reports personal fees from Miami Device Solutions, other from Arthrex, other from Smith and Nephew, other from Wright/Tornier, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schairer WW, Nwachukwu BU, Lyman S, et al. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg 2015;24:91-7. [Crossref] [PubMed]
- Hyun YS, Huri G, Garbis NG, et al. Uncommon indications for reverse total shoulder arthroplasty. Clin Orthop Surg 2013;5:243-55. [Crossref] [PubMed]
- Levy J, Frankle M, Mighell M, et al. The Use of the Reverse Shoulder Prosthesis for the Treatment of Failed Hemiarthroplasty for Proximal Humeral Fracture. J Bone Joint Surg Am 2007;89:292-300. [Crossref] [PubMed]
- Raiss P, Edwards TB, da Silva MR, et al. Reverse Shoulder Arthroplasty for the Treatment of Nonunions of the Surgical Neck of the Proximal Part of the Humerus (Type-3 Fracture Sequelae). J Bone Joint Surg Am 2014;96:2070-6. [Crossref] [PubMed]
- Shukla DR, Lee J, Mangold D, et al. Reverse Shoulder Arthroplasty With Proximal Humeral Replacement for the Management of Massive Proximal Humeral Bone Loss. J Shoulder Elb Arthroplasty 2018;2:247154921877984 [Crossref]
- Wright TW, Cofield RH. Humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am 1995;77:1340-6. [Crossref] [PubMed]
- Worland RL, Kim DY, Arredondo J. Periprosthetic humeral fractures: Management and classification. J Shoulder Elbow Surg 1999;8:590-4. [Crossref] [PubMed]
- Mineo GV, Accetta R, Franceschini M, et al. Management of shoulder periprosthetic fractures: Our institutional experience and review of the literature. Injury 2013;44:S82-5. [Crossref] [PubMed]
- Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res 2016;102:S33-43. [Crossref] [PubMed]
- Wutzler S, Laurer HL, Huhnstock S, et al. Periprosthetic humeral fractures after shoulder arthroplasty: operative management and functional outcome. Arch Orthop Trauma Surg 2009;129:237-43. [Crossref] [PubMed]
- Kumar S, Sperling JW, Haidukewych GH, et al. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am 2004;86-A:680-9. [Crossref] [PubMed]
- Andersen JR, Williams CD, Cain R, et al. Surgically Treated Humeral Shaft Fractures Following Shoulder Arthroplasty J Bone Joint Surg Am 2013;95:9-18. [Crossref] [PubMed]
- Ohl X, Bonnevialle N, Gallinet D, et al. How the greater tuberosity affects clinical outcomes after reverse shoulder arthroplasty for proximal humeral fractures. J Shoulder Elbow Surg 2018;27:2139-44. [Crossref] [PubMed]
- Poltaretskyi S, Chaoui J, Mayya M, et al. Prediction of the pre-morbid 3D anatomy of the proximal humerus based on statistical shape modelling. Bone Joint J 2017;99-B:927-33. [Crossref] [PubMed]
- Budge MD, Moravek JE, Zimel MN, et al. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg 2013;22:739-44. [Crossref] [PubMed]
- Gebrelul A, Green A, Schacherer T, et al. Periprosthetic humerus fractures: classification, management, and review of the literature. Ann Joint 2018;3:49. [Crossref]
- Owens CJ, Sperling JW, Cofield RH. Utility and complications of long-stem humeral components in revision shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:e7-12. [Crossref] [PubMed]
- Sommacal R, Bloch HR, Ghidelli A, et al. Comminuted periprosthetic humeral fracture after reverse shoulder prosthesis. Chir Organi Mov 2009;93:S83-7. [PubMed]
- Sewell MD, Kang SN, Al-Hadithy N, et al. Management of peri-prosthetic fracture of the humerus with severe bone loss and loosening of the humeral component after total shoulder replacement. J Bone Joint Surg Br 2012;94:1382-9. [Crossref] [PubMed]
- Martinez AA, Calvo A, Cuenca J, et al. Internal Fixation and Strut Allograft Augmentation for Periprosthetic Humeral Fractures. J Orthop Surg (Hong Kong) 2011;19:191-3. [Crossref] [PubMed]
- Stephens SP, Paisley KC, Giveans MR, et al. The effect of proximal humeral bone loss on revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:1519-26. [Crossref] [PubMed]
- Sanchez-Sotelo J, Wagner ER, Sim FH, et al. Allograft-Prosthetic Composite Reconstruction for Massive Proximal Humeral Bone Loss in Reverse Shoulder Arthroplasty J Bone Joint Surg 2017;99:2069-76. [Crossref] [PubMed]
- Chacon A, Virani N, Shannon R, et al. Revision Arthroplasty with Use of a Reverse Shoulder Prosthesis-Allograft Composite J Bone Joint Surg Am 2009;91:119-27. [Crossref] [PubMed]
- Cannon CP, Paraliticci GU, Lin PP, et al. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg 2009;18:705-10. [Crossref] [PubMed]
- Abdeen A, Healey JH. Allograft-Prosthesis Composite Reconstruction of the Proximal Part of the Humerus: Surgical Technique. J Bone Joint Surg Am 2010;92:188-96. [Crossref] [PubMed]
- Martinez AA, Bejarano C, Carbonel I, et al. The treatment of proximal humerus nonunions in older patients with reverse shoulder arthroplasty. Injury 2012;43:S3-6. [Crossref] [PubMed]
Cite this article as: Krueger VS, Santaella B, Green A, Paxton ES. Pitfalls of revision reverse replacement part II: dealing with humeral bone loss and periprosthetic fractures. Ann Joint 2018;3:101.


