Clinical articular cartilage repair—an up to date review
Introduction
Articular cartilage is regarded as a troublesome tissue due to its poor ability to self-repair post-trauma (1,2). Main causes are that cartilage is lacking blood vessels, nerves and lymphatic tissue (1,2). Hence, the repair is difficult to induce. Furthermore, when studying the possibilities to repair a damage cartilage surface, one has to look at three types of cartilage damages:
- Pure acute traumatic defects with more or less healthy surrounding cartilage;
- Degenerative lesions seen after repeated trauma where surrounding cartilage is of less good quality in an otherwise healthy joint;
- Loss of cartilage due to pathology of the cells and matrix; osteoarthritis (OA). OA is an organ disease.
It is a gliding scale of tissue destruction where an isolated cartilage defect may remain stationary without progress while other injuries progress via a pre-osteoarthritic state into a full blown OA. Concomitant injuries like meniscal loss and ACL injuries may fasten such an OA development.
When a cartilage area has been damage an impairment of the joint function could develop, a disturbed joint homeostasis. Symptoms like localized pain, locking phenomena and loss of motions may appear. With diagnostics used like MRI evaluations cartilage lesions may be found and if regarded as being the cause of the pain situation, the surgeon suggests an arthroscopy evaluation and subsequent repair. With the MRI, bone marrow lesions (BMLs) in conjunction with local chondral and osteochondral defects may be detected. BMLs and such cartilage defects are interconnected and play key roles in knee cartilage volume loss which indicates that both should be considered targets for intervention (3).
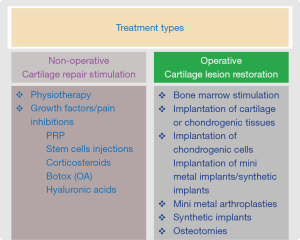
Treatment options may be either non-operative (cartilage repair stimulators) or operative (cartilage repair restoration) (see Figure 1).
Classification of the lesions
It is useful to look upon the cartilage lesions to treat related to the existing depth of the lesions. The ICRS classification system for chondral and osteochondral lesions is based on the depth (4). In general, grade 1 and 2 are defects with a depth less than 50% of the cartilage thickness. Such defects might be treated with debridement alone if the lesions are suspected to produce mechanical symptoms. Deeper lesions are all the defects with a depth more than 50% of the cartilage thickness including also cartilage blisters. Defects that penetrate the subchondral bone are grade 4 (4). All grade 3 and 4 defects are lesions that most often may need a cartilage repair operation, lesion restoration.
In this review we will look at the treatment alternatives used today related to the sizes and depth of symptomatic cartilage lesions.
Non-operative treatments
Cartilage lesions are seldom the only cause of disability and many cartilage lesions are also asymptomatic. Subsequently, if the cause of disability is uncertain a period of non-operative therapy may be wise to test. Below are some non-operative alternatives that are sometimes used before decision of surgery is taken.
Physiotherapy
There are no studies to compare just physiotherapy with operative cartilage repair. Physiotherapy is, however, an important adjunct to operative repairs. Many athletes being very active in the trainings and rehabs have asymptomatic lesions (5) and when finishing their career, symptoms appear as they do less physio-training.
However, interestingly Helmark et al. (6) showed that exercise could induce an increase in both intraarticular and peri-synovial concentrations of IL-10 in a group of human females with knee OA. The finding suggests a positive effect of exercise on a chondroprotective anti-inflammatory cytokine response in patients with knee OA and might contribute to explain the beneficial effect that exercise has on OA and possibly also on cartilage local repairs.
PRP (platelet rich plasma) and stem cells injections
Both types of injections are thought to stimulate the repair of damaged cartilage. Most clinical studies have been done on OA, being an organ disease. None of the techniques have so far been able to show that local cartilage defects have been fully repaired. However, in a recent review Shi et al. (7) did a search of the PubMed, EMBASE, and Cochrane databases to identify studies involving biologic therapy for osteoarthritis or osteochondral defects. Only Level I–III clinical trials with at least 3-month follow-up were included. A total of 21 PRP studies were included in the study. All PRP studies showed clinical improvement with PRP therapies related to outcomes in patient satisfaction, pain, and function (7). Furthermore, there were 7 of the 9 studied MSC studies that showed clinical improvement. The one PRP study that had a 2nd look arthroscopy reported increased cartilage repair tissue with PRP. All 8 MSC studies with follow-up MRI and all 7 MSC studies with 2nd look arthroscopy showed improvement in cartilage repair in terms of coverage, fill of the defect, and/or firmness of the new cartilage. The authors concluded that of the two treatments, MSC provides more significant disease modifying effect (7). Still, the efficacy of PRP therapy remains unpredictable due to the highly heterogeneous nature of reported studies and the variable composition of the PRP preparations. It is similar with MSc’s treatment as the source of MSc’s varies and also with those cells highly varied quality of reported studies.
Corticosteroids
Post-trauma OA could be induced at the time of joint injury resulting early on in substantial matrix changes. However, Lattermann et al. (8) could show that early intervention with corticosteroids was able to affect biomarkers of cartilage breakdown compared to control (saline). Corticosteroids are mainly used in OA with synovitis but the study by Latterman et al. (8) may turn a focus to also to use corticosteroids to prevent post trauma cartilage damage.
Botox
Botox has been of interest to treat pain related to OA but there are no thoughts that Botox injections could contribute to repair of local defects. In a recent RCT (9), there were no significant differences between botulinum toxin A injections and placebo in reducing average pain score at week 8 compared with baseline in patients with knee OA. No safety concerns were identified (9).
Hyaluronic acids and glucosamine/chondroitin sulphates
In a recent review Gallagher et al. (10) concluded that for patients with or at risk for osteoarthritis, the use of glucosamine and chondroitin sulfate may serve as non-operative means to protect joint cartilage and delay osteoarthritis progression. Hyaluronic acid injections showed variable efficacy, while NSAIDs and vitamins E and D showed no effect on osteoarthritis progression (10). Most often those treatments are used for the pain relief, especially, the hyaluronic acid injections.
Operative treatments
Bone marrow stimulation (BMS) techniques
The earliest techniques in articular cartilage repair are based on the formation of a hematoma at the defect site with the migration of stem cells/chondrogenic progenitor cells from the bone marrow.
This process produces a fibrocartilage repair tissue to more or less fill the defect. Microfracture technique (MFX) was introduced by Steadman et al. in 1999 (11) and MFX has since then been the dominating first of choice cartilage repair technique the last 30 years.
However, animal studies have shown that micro fracturing does not reach deep enough to reach large vessels in the bone marrow (12). When reaching large vessels, more pericytes/mesenchymal cells could be attracted to migrate into the defect area to start a repair process. Deep drilling has shown to produce more repair filling than microfracture treatment and new techniques, so called nano-drillings are now in use to induce a stronger repair response (12).
Different BMS techniques are also used together with scaffolds. Such enhanced BMSs are now often combined with modifications of nano-drilling such an AMIC (autologous matrix induced chondrogenesis) with collagen type I–III membrane (13) and hyaluronic acid based membranes (Hyaff-11) (14). The Hyaff-11 membranes have also been combined with bone marrow aspirate concentrates (BMAC). Gobbi and Whyte (14) could show that repair of chondral injury using a hyaluronic acid-based scaffold with activated bone marrow aspirate concentrate provides better clinical outcomes and more durable cartilage repair at 5 year’s follow-up compared with microfracture.
There are also the biphasic membranes with a mixture of collagen and hydroxyapatite with magnesium (15). The biphasic implant has been tested against MFX in a randomized multi-center study and was significantly better than MFX treating osteochondral defects and sports active patient (15). Another augmented BMS technique is a liquid bioscaffold, an injection of a thermogel into the microfractured area (16). The thermogel consists of a mixture of chitosan and venous blood and when stabilized in the lesion area, it becomes like a ¨superclot¨ for the invading cells from the bone marrow (16). In a RCT with the augmented gel treatment versus MFX alone, the gel technique showed statistically superiority with the gel in the structural repair tissue regarding MRI and histology at 2 and 5 years (16,17).
There are also bi-phasic osteochondral scaffolds made out of corals. In a recent animal study, Kon et al. showed that although native coral is an interesting material for bone repair, as a stand-alone material implant, it did not induce hyaline-like cartilage. Mechanical modification with drilled channels and impregnation of HA within the coral pores enhanced the scaffold’s cartilage regenerative potential (18). They have also used the improved scaffold in a mini human trial and found a significant clinical improvement at the 12-month follow-up. Moreover, MRI findings revealed graft integration with good bone and cartilage formation (19).
Chondrogenic tissue based repairs
Perichondrium-based repairs
Perichondrium based repairs were popular from 1990–2000. However, due to problems with ossifications in the graft and due to such a complication in the long run less good results The clinical use is low today. Best results were seen in young patients (20).
Periosteum-based repairs
Periosteum has with its cambium cell-layer chondrogenic potential that makes it possible to repair cartilage lesions. However, the potential chondrogenic cell layer diminish fast with increasing age and already around the age of 20, the patients ability to be repaired with their periosteum has drastically declined. Periosteum is still an option when to repair large osteochondral injuries in an acute traumatized joint in an individual before closures of epiphyses while additional cell transplantation better fit the older patient (21,22).
Autologous osteochondral grafts
These implants were made popular during the 90ies by Bobić (23) and Hangody et al. (24). Still in large use but has lost in popularity the last years. A single and up to 2–3 grafts are useful for athletes wanting a relative fast rehab and receiving a fast stable cartilage graft. The technique is technically demanding when to treat large defects trans-arthroscopically and there is a risk of incongruity at the bone cartilage interface. There is also a risk of graft donor site morbidity (25). Solheim et al. (26) showed that MFX articular cartilage repairs failed more often and earlier than the osteochondral repairs, both in the whole cohort and in a subgroup of patients matched for age and size of treated lesion, indicating that the osteochondral graft repair is the more durable.
Osteochondral allografts
The use of allografts has been successful with good results up to 20 years post-surgery (27). The backsides are lack of donors and risk for disease transmission after implantation (28). There is also the possibility to use decellularized osteochondral allografts. Such implants are then available of the shelves and will give no joint morbidity. Those implants are composed of donated human decellularized hyaline cartilage and cancellous bone. However, in comparison to the osteochondral fresh allografts, those decellularized grafts show a high failure rate. Johnson et al. (29) showed that in a study of 34 patients with such implants, ten patients (29%) required revision surgery with removal of the implant. Implant survivorship was 61% at 2 years. Farr et al. (30) had a similar experience with 23 of the 32 knees (72%) that were considered failures and implant survivorship was only 19.6% at 2 years.
Chondrogenic cell implantations
The chondrocyte is responsible for the matrix production and such a cell seems the ideal cell to use for cartilage engineering (31). The first clinical use of chondrocytes for clinical cartilage repair was done in Gothenburg, Sweden in 1987 (31). There exist today long-term results up to 20 years with good results based on the 1st generation ACI with chondrocytes in suspension implanted under a periosteal membrane (32-35).
Since that time more than 30 years ago, we have now four generations of ACI:
- 1st generation ACI: Chondrocytes in suspension injected under a living periosteal membrane;
- 2nd generation ACI with cells in suspension injected under a collagen membrane;
- 3rd generation of ACI with cells either grown on a surface carrier cells grown in a porous matrix/scaffold (see Figure 2A,B,C);
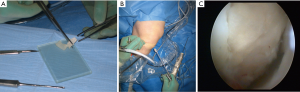 Figure 2 The steps of cart8ilage lesion preparation and implantation of a cell seeded hyaluronic graft (Hyaff-11) are presented. (A) A chondrocyte seeded graft (Hyaff-11 scaffold) is sized into right lesion size before implantation; (B) the chondrocyte seeded graft is implanted with a plain grasper transarthroscopic through the portal; (C) the chondrocyte seeded graft has been put into place in the lesion area and secured with a layer of fibrin glue.
Figure 2 The steps of cart8ilage lesion preparation and implantation of a cell seeded hyaluronic graft (Hyaff-11) are presented. (A) A chondrocyte seeded graft (Hyaff-11 scaffold) is sized into right lesion size before implantation; (B) the chondrocyte seeded graft is implanted with a plain grasper transarthroscopic through the portal; (C) the chondrocyte seeded graft has been put into place in the lesion area and secured with a layer of fibrin glue. - 4th generation ACI is when chondrocytes are in different ways implanted as one stage procedures.
ACI has as a procedure been studied in up to date 18 randomized studies (36-53).
In 8 studies, different generations of ACI were compared against MFX (40,45-51). In 6/8 of those studies, ACI was significantly better clinically than MFX marrow stimulation in different parameters studied (45-48,50,51).
The latest study is the SUMMIT trial where an ACI 3rd generation, a cell carrier (MACI) is compared with MFX (54). At 2, 3 and 5 years post-surgery, the ACI had significantly better outcomes in co-primary endpoints KOOS and function compared with MFX (51,54).
It is also worth to mention the 4th generation ACI. One of those techniques is the CAIS (cartilage autograft implantation system) where cartilage is harvested and crushed into small fragments, placed on a resorb able membrane and implanted into the defect area covered by fibrin glue. Two randomized studies have shown statistical superiority with the cartilage fragments versus MFX treatments (47,48). Same technology has recently been developed with juvenile allogeneic fragments.
In the 4th generation group, also two new one stage procedures are placed. The Instruct technology isolates chondrocytes direct in the OR and mixes those cells with autologous direct harvested mesenchymal stem cells (55). The Impact technology direct isolates chondrons (chondrocytes with pericellular matrix) and mix them with allogeneic mesenchymal stem cells (56).
Non-biological implants
Mini-metal arthroplasties and synthetic implants
Even the different available biological alternatives are not successful every time when used. Some patients are poor responders to local biological repairs with the techniques above. Some of them if located in a medial or lateral femoral compartment could be improved by unloading osteotomies but still some remain disabled not being candidates for larger joint arthroplasties. There are now custom made metal implants that can be used for local repairs as well as synthetic min-implants. Still, there is little clinical experience with those types of implants but we will see more of them in the future (57,58).
Unloading osteotomies
As an extra support for the repair tissue, an unloading osteotomy or unloading brace can be used If or not there is a need depends on:
- Lesion size;
- Degree of malalignment;
- Instability;
- Weight.
For the knee, the osteotomy can be done either as closing wedge or open wedge osteotomy. The opening wedge osteotomy influences the tibial slope. A reduced tibial slope increase the stability of an ACL-deficient knee and unload posterior located femoral condyle cartilage lesions. An increased tibial slope is good when posterior cruciate ligament insufficiency exists and an increased slope also unloads anterior located cartilage lesions (59).
Summary and conclusions
What we have learned during the last 50 years is that all types of cartilage repairs are slow and a maturation process of the repair can be seen up to 4 years post-surgery. Knowing that it takes up to 20 years before the cartilage in a child reaches the morphology characteristics of adult cartilage, we have to better understand the need for the long maturation time for our induced repair.
More studies on the use of one stage repairs with mesenchymal stem cells of different origins will be seen. For all types of existing repair methods, the postoperative rehabilitation is the weakest part of the treatment. Not enough knowledge and evidence exist of what rehab is the best. There is a tendency for faster rehab with early weight bearing. In this review on cartilage repair, the focus has been on the local trauma defects to be treated. However, there is an enormous interest to intervene early on OA. In a study by Spahn and Hofmann (60), the authors looked upon 115 patients with focal cartilage lesions of the medial knee compartment. A follow-up was performed 10 years after the operation to determine the rate of arthroplasty conversion and to evaluate associated factors. In a total of 35 cases an arthroplasty was needed (30.4%). Among the significant risk factors for OA progression were (60):
- Higher patient age;
- Female gender;
- Overweight or obesity;
- Severity of meniscal damage.
Interestingly, the most important risk factor was the occurrence and the extent of tibial cartilage defects. For the cartilage repair surgeon the task is not only to give patient pain relief but also to find treatments to hinder a progress of the cartilage lesions into osteoarthritis.
Finally, I present here an easy to use algorithm for how to choose repair techniques. Within each group, there exist several different repair methods but no science to tell that one technique is better than other ones.
- BMS for small defects <1 cm2;
- Augmented BMS for small-medium sized defect 1–3 cm2;
- Augmented BMS is also an alternative for re-operations in such defects if a simple BMS has been done before;
- Cell based treatments for large defects >3 cm2;
- Cell based treatments for re-operations;
- Allografts for extra-large defects and condylar replacements;
- Synthetic or min-metal implants when patients are not responding to biological treatments;
- Unloading osteotomies are useful in combination with local repairs when the lesions are large, when the lesions are uncontained and when malalignment exists.
Available cartilage repair techniques can induce a good quality repair tissue that gives the patient long-term pain relief and functional recovery. Still, we have not seen any repair technique being able to induce a full regeneration of the traumatized cartilage area.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.11.09). MB reports personal fees from Verigen, personal fees from Anika therapeutics, personal fees from Finceramica, personal fees from Episurf AB, personal fees from Journal CARTILAGE, outside the submitted work.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am 1982;64:460-66. [Crossref] [PubMed]
- Sokoloff L. Repair mechanisms of articular cartilage. J Am Podiatry Assoc 1982;72:228-32. [Crossref] [PubMed]
- Dore D, Martens A, Quinn S, et al. Bone marrow lesions predict site-specific cartilage defect development and volume loss: a prospective study in older adults. Arthritis Res Ther 2010;12:R222. [Crossref] [PubMed]
- Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 2003;85-A:58-69. [Crossref] [PubMed]
- Flanigan DC, Harris JD, Trinh TQ, et al. Prevalence of chondral defects in athletes' knees: a systematic review. Med Sci Sports Exerc 2010;42:1795-801. [Crossref] [PubMed]
- Helmark IC, Mikkelsen UR, Børglum J, et al. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res Ther 2010;12:R126. [Crossref] [PubMed]
- Shi WJ, Tjoumakaris FP, Lendner M, et al. Biologic injections for osteoarthritis and articular cartilage damage: can we modify disease? Phys Sportsmed 2017;45:203-23. [Crossref] [PubMed]
- Lattermann C, Jacobs CA, Proffitt Bunnell M, et al. A Multicenter Study of Early Anti-inflammatory Treatment in Patients With Acute Anterior Cruciate Ligament Tear. Am J Sports Med 2017;45:325-33. [Crossref] [PubMed]
- McAlindon TE, Schmidt U, Bugarin D, et al. Osteoarthritis Efficacy and safety of single-dose onabotulinumtoxinA in the treatment of symptoms of osteoarthritis of the knee: results of a placebo-controlled, double-blind study. Osteoarthritis Cartilage 2018;26:1291-99. [Crossref] [PubMed]
- Gallagher B, Tjoumakaris FP, Harwood MI, et al. Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med 2015;43:734-44. [Crossref] [PubMed]
- Steadman JR, Rodkey WG, Briggs KK, et al. The microfracture technic in the management of complete cartilage defects in the knee joint. Orthopade 1999;28:26-32. [PubMed]
- Chen H, Hoemann CD, Sun J, et al. Depth of subchondral perforation influences the outcome of bone marrow stimulation cartilage repair. J Orthop Res 2011;29:1178-84. [Crossref] [PubMed]
- Benthien JP, Behrens P. Autologous Matrix-Induced Chondrogenesis (AMIC): Combining Microfracturing and a Collagen I/III Matrix for Articular Cartilage Resurfacing. Cartilage 2010;1:65-68. [Crossref] [PubMed]
- Gobbi A, Whyte GP. One-Stage Cartilage Repair Using a Hyaluronic Acid-Based Scaffold With Activated Bone Marrow-Derived Mesenchymal Stem Cells Compared With Microfracture: Five-Year Follow-up. Am J Sports Med 2016;44:2846-54. [Crossref] [PubMed]
- Kon E, Filardo G, Brittberg M, et al. A multilayer biomaterial for osteochondral regeneration shows superiority vs microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg Sports Traumatol Arthrosc 2018;26:2704-15. [Crossref] [PubMed]
- Stanish WD, McCormack R, Forriol F, et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am 2013;95:1640-50. [Crossref] [PubMed]
- Shive MS, Stanish WD, McCormack R, et al. BST-CarGel® Treatment Maintains Cartilage Repair Superiority over Microfracture at 5 Years in a Multicenter Randomized Controlled Trial. Cartilage 2015;6:62-72. [Crossref] [PubMed]
- Kon E, Filardo G, Robinson D, et al. Osteochondral regeneration using a novel aragonite-hyaluronate bi-phasic scaffold in a goat model. Knee Surg Sports Traumatol Arthrosc 2014;22:1452-64. [Crossref] [PubMed]
- Kon E, Robinson D, Verdonk P, et al. A novel aragonite-based scaffold for osteochondral regeneration: early experience on human implants and technical developments. Injury 2016;47:S27-S32. [Crossref] [PubMed]
- Homminga GN, Bulstra SK, Bouwmeester PS, et al. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br 1990;72:1003-7. [Crossref] [PubMed]
- Niedermann B, Boe S, Lauritzen J, et al. Glued periosteal grafts in the knee. Acta Orthop Scand 1985;56:457-60. [Crossref] [PubMed]
- Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res 1991;9:465-76. [Crossref] [PubMed]
- Bobić V. Arthroscopic osteochondral autograft transplantation in anterior cruciate ligament reconstruction: a preliminary clinical study. Knee Surg Sports Traumatol Arthrosc 1996;3:262-4. [Crossref] [PubMed]
- Hangody L, Kish G, Kárpáti Z, et al. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc 1997;5:262-7. [Crossref] [PubMed]
- Andrade R, Vasta S, Pereira R, et al. Knee donor-site morbidity after mosaicplasty – a systematic review. J Exp Orthop 2016;3:31. [Crossref] [PubMed]
- Solheim E, Hegna J, Inderhaug E. Long-Term Survival after Microfracture and Mosaicplasty for Knee Articular Cartilage Repair: A Comparative Study Between Two Treatments Cohorts. Cartilage 2018;1947603518783482 [Epub ahead of print]. [PubMed]
- Raz G, Safir OA, Backstein DJ, et al. Distal Femoral Fresh Osteochondral Allografts: Follow-up at a Mean of Twenty-two Years. J Bone Joint Surg Am 2014;96:1101-7. [Crossref] [PubMed]
- Squillace DM, Zhao Z, Call G, et al. Viral Inactivation of Human Osteochondral Grafts with Methylene Blue and Light. Cartilage 2014;5:28-36. [Crossref] [PubMed]
- Johnson CC, Johnson DJ, Garcia GH, et al. High Short-Term Failure Rate Associated With Decellularized Osteochondral Allograft for Treatment of Knee Cartilage Lesions. Arthroscopy 2017;33:2219-27. [Crossref] [PubMed]
- Farr J, Gracitelli GC, Shah N, et al. High Failure Rate of a Decellularized Osteochondral Allograft for the Treatment of Cartilage Lesions. Am J Sports Med 2016;44:2015-22. [Crossref] [PubMed]
- Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889-95. [Crossref] [PubMed]
- Peterson L, Vasiliadis HS, Brittberg M, et al. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med 2010;38:1117-24. [Crossref] [PubMed]
- Nawaz SZ, Bentley G, Briggs TW, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am 2014;96:824-30. [Crossref] [PubMed]
- Rosa D, Balato G, Ciaramella G, et al. Long-term clinical results and MRI changes after autologous chondrocyte implantation in the knee of young and active middle aged patients. J Orthop Traumatol 2016;17:55-62. [Crossref] [PubMed]
- Ogura T, Mosier BA, Bryant T, et al. A 20-Year Follow-up After First-Generation Autologous Chondrocyte Implantation. Am J Sports Med 2017;45:2751-61. [Crossref] [PubMed]
- Horas U, Pelinkovic D, Herr G, et al. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am 2003;85-A:185-92. [Crossref] [PubMed]
- Schneider U, Andereya S. First results of a prospective randomized clinical trial on traditional chondrocyte transplantation vs CaReS-Technology. Z Orthop Ihre Grenzgeb 2003;141:496-97. [PubMed]
- Bentley G, Biant LC, Carrington RW, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br 2003;85:223-30. [Crossref] [PubMed]
- Visna P, Pasa L, Cizmár I, et al. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques--a randomized controlled study. Acta Chir Belg 2004;104:709-14. [Crossref] [PubMed]
- Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 2004;86-A:455-64. [Crossref] [PubMed]
- Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br 2005;87:640-5. [Crossref] [PubMed]
- Dozin B, Malpeli M, Cancedda R, et al. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med 2005;15:220-6. [Crossref] [PubMed]
- Gooding CR, Bartlett W, Bentley G. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee 2006;13:203-10. [Crossref] [PubMed]
- Zeifang F, Oberle D, Nierhoff C, et al. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med 2010;38:924-33. [Crossref] [PubMed]
- Basad E, Ishaque B, Bachmann G, et al. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc 2010;18:519-27. [Crossref] [PubMed]
- Vanlauwe J, Saris DB, Victor J, et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med 2011;39:2566-74. [Crossref] [PubMed]
- Cole BJ, Farr J, Winalski CS, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med 2011;39:1170-9. [Crossref] [PubMed]
- Spalding T, Almqvist F, Brittberg M, et al. The CAIS project: European multicenter randomized controlled pilot study of a one stage procedure procedure for cell-based cartilage repair. Bone and Joint Publishing, 2011;93-B.
- Lim HC, Bae JH, Song SH, et al. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res 2012;470:2261-7. [Crossref] [PubMed]
- Crawford DC, DeBerardino TM, Williams RJ 3rd. NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am 2012;94:979-89. [Crossref] [PubMed]
- Saris D, Price A, Widuchowski W, et al. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Two-Year Follow-up of a Prospective Randomized Trial. Am J Sports Med 2014;42:1384-94. [Crossref] [PubMed]
- Akgun I, Unlu MC, Erdal OA, et al. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg 2015;135:251-63. [Crossref] [PubMed]
- Clavé A, Potel JF, Servien E, et al. Third-generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2-year randomized trial. J Orthop Res 2016;34:658-65. [Crossref] [PubMed]
- Brittberg M, Recker D, Ilgenfritz J, et al. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am J Sports Med 2018;46:1343-51. [Crossref] [PubMed]
- Hendriks J, Verdonk P, Widuchowski W, et al. First clinical experience with INSTRUCT - a single surgery, autologous cell based technology for cartilage repair. ICRS abstract proceedings 2013. Electronic Poster P187. Available online: https://cposter.ctimeetingtech.com/get/pdf/icrs/5931/
- de Windt TS, Vonk LA, Slaper-Cortenbach ICM, et al. Allogeneic MSCs and Recycled Autologous Chondrons Mixed in a One-Stage Cartilage Cell Transplantation: A First-in-Man Trial in 35 Patients. Stem Cells 2017;35:1984-93. [Crossref] [PubMed]
- Stålman A, Sköldenberg O, Martinez-Carranza N, et al. No implant migration and good subjective outcome of a novel customized femoral resurfacing metal implant for focal chondral lesions. Knee Surg Sports Traumatol Arthrosc 2018;26:2196-204. [Crossref] [PubMed]
- Nathwani D, McNicholas M, Hart A, et al. Partial Resurfacing of the Knee with the BioPoly Implant: Interim Report at 2 Years. JB JS Open Access 2017;2:e0011.
- Rodner CM, Adams DJ, Diaz-Doran V, et al. Medial opening wedge tibial osteotomy and the sagittal plane: the effect of increasing tibial slope on tibiofemoral contact pressure. Am J Sports Med 2006;34:1431-41. [Crossref] [PubMed]
- Spahn G, Hofmann GO. Focal cartilage defects within the medial knee compartment. predictors for osteoarthritis progression. Z Orthop Unfall 2014;152:480-8. [PubMed]
Cite this article as: Brittberg M. Clinical articular cartilage repair—an up to date review. Ann Joint 2018;3:94.