Symptoms and physical findings of early knee osteoarthritis
Introduction
There is an increasing need on identifying symptoms and physical findings of early knee osteoarthritis (KOA), which is never recovered once destroyed (1). More than 21 million people suffered from osteoarthritis (OA) and elderly Americans aged 70 or older have some degree of radiographic knee OA in the U.S. (2-4). In Japan, a rapid super-ageing society, KOA affects approximately 25 million people, estimated 48.2% and 51.6% in the age groups 70–79 and >80 years in men, and 71.9% and 80.7% in women, respectively (5). Symptomatic KOA deteriorates activity of daily living (ADL), impairs the health-related quality of life (HRQoL), and consumes a significant amount of healthcare resources. A systemic review showed that the total annual average direct and indirect costs per patient with OA varied from US$1442 to US$21,335 and from US$238 to US$29,935, respectively, both in USA (6). In addition, joint replacement surgeries are most often indicated for knee and hip OA; 905,000 knees and hips were replaced in 2009 at a cost of $42.3 billion (7). World Health Organization (WHO) estimates that the number of people over 60 years will rise 900 million to 2 billion between 2015 and 2050, and address that OA is one of the greatest causes of disability and comprehensive public health action will require fundamental shifts in how we think about ageing and health (8).
There are several established definitions of OA and classification criteria of KOA (9-11), however, it is more complicated to diagnose the early KOA than the established KOA. To identify it clinically relevant, and to contribute to classifying patients for clinical trials, Luyten et al. proposed the criteria for early KOA as follows (12):
- Pain in the knee.
- Radiographic grading as classified by Kellgren-Lawrence grade < II.
- Structural findings proven by arthroscopy and/or MRI:
- Arthroscopic findings of cartilage lesions as defined by International Cartilage Repair Society (ICRS) I–IV in two compartments or II–IV in one compartment (13);
- MRI findings of cartilage lesions defined as Whole-Organ Magnetic Resonance Imaging (WORMS) 3–6 (14), or Boston Leeds Osteoarthritis Knee Score (BLOKS) grades 2–3 (15), meniscus injury defined by BLOKS grades 3 and 4, or bone marrow lesion.
These criteria including pain and radiographic findings might be indeed easily used for clinicians, however structural criteria including arthroscopic and/or MRI findings might be sometimes difficult clinically and epidemiologically in terms of invasive test and high-demanded costs. Therefore, we propose the usage of non-invasive, fast, easy-to-use and safe ultrasonography (US) in addition to standard radiographs to detect early KOA.
In this paper, we demonstrate clinical manifestation of early KOA detected by US and standard radiographs and characterize them for early detection of KOA. In addition, we address the importance of recognition of symptoms and physical findings in the pre-stage of early KOA, namely super-early KOA to prevent from proceeding to early KOA.
Methods
The Ethics Committee of Shimane University School of Medicine approved the present study, and all participants provided written informed consent. Participants were recruited at residential health examinations held in Shimane prefecture from 2011 through 2014. In total, 1,060 participants (2,120 knees) were recruited. Measurements of height and weight were performed on site. They answered questionnaires about their musculoskeletal symptoms, especially of knee joints, their past and present medical history, occupation, family history, and health-related quality of life. And they underwent a radiographic examination, muscle power test, and US.
Clinical manifestations
The severity of pain was measured by a visual analog scale (VAS, 0 to 100). The clinical symptoms, activities and health conditions were evaluated by the Japanese Knee Osteoarthritis Measure (JKOM) score (13). The JKOM score is a patient-based, self-answered evaluation score that consists of four subscales: pain and stiffness (0 to 32), ADLs (0 to 40), social activities (0 to 20), and general health conditions (0 to 8) with 100 points as the maximum score. The JKOM score is higher in KOA patients with more pain and physical disability, and this evaluation modality have been shown to be a reliable and stable outcomes measure for studies of the clinical outcomes of Japanese subjects with knee OA (16). Statistical evaluation and comparison have also proven its enough reliability and validity with other health-related scales, such as the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) (16).
Radiographs and US
One of the authors (HT, who has 10 years of experience as orthopedists) categorized radiographs of both knees in the standing position based on the Kellgren-Lawrence grade (KLG) (17). The knees were classified as follows:
- KLG 0 (no radiographic features of OA);
- KLG 1 [doubtful joint space narrowing (JSN) and possible osteophytic lipping];
- KLG 2 (definite osteophytes and/or possible JSN);
- KLG 3 (multiple osteophytes, definite JSN, sclerosis, possible bony deformity), and;
- KLG 4 (large osteophytes, marked JSN, severe sclerosis and definite bony deformity).
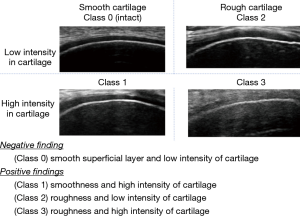
Two of the authors (SK and NK who have 5 years of experience in ultrasonographic evaluation of early KOA) evaluated all ultrasound scans. The subjects were examined sitting on the table with a flexed knee in the deep flexed position. The longitudinal sagittal scan to the distal end of the midline medial and lateral condyle were obtained by a linear transducer probe (14–16 MHz, Avius®; HITACHI ALOKA Ltd., Tokyo, Japan). The cartilage was subjectively evaluated using 4 classifications based on the US findings of the condition of the superficial layers (smooth or rough) and the intensity of cartilage (monotonous anechoic or hyper echogenicity) as follows (15): class 0 (smooth surface and monotonous echogenicity), class 1 (smooth surface and increased echogenicity), class 2 (rough surface and monotonous echogenicity), and class 3 (rough surface and hyper echogenicity). Class 0 is regarded as echo-negative, and Class 1 or more as echo-positive findings (Figure 1). Statistical evaluation and comparison with arthroscopic findings have been shown enough reliability and validity of the US (18-21).
Muscle strength
Finally, we (YU and HT) evaluated maximum knee extension muscle strength of the subjects by the Locomo Scan® (ALCARE Co., Ltd., Tokyo, Japan) (22). The subjects extended their knee joint by putting their knee joint on the knee-holding part of the Locomo Scan®, with the load pressure applied to the Locomo Scan® in the popliteal region at this time measured and displayed as the isometric knee extension strength (quadriceps muscle strength). The maximum voluntary contraction of the quadriceps muscle was measured for each leg with the Locomo Scan® and recorded the better value of the two measured values. The value divided by weight was used as the extension muscle strength for statistical analysis. The average muscle strengths in total men and women were calculated as reference.
Statistical analysis
The means and standard deviations (SDs) were calculated for weight, height, BMI, and maximum knee extension muscle strength of each leg per weight, in each category.
Results were analyzed using one-way analysis of variance, multiple comparison by scheffe method, and logistic regression analysis from SPSS 22, IBM®. A P value ≤0.05 was considered statistically significant.
Results
Subject characteristics
The present study was participated by 1,090 individuals (345 men, 745 women; mean age: 71 years, 40–91 years). The means (range) of weight, height, and body mass index (BMI) in the present study are 153.8 cm (129.3–181.5 cm), 54.3 kg (28.2–98.6 kg), and 22.9 (14.2–35.9), respectively. Number of the participants classified by sex, KLG and US class are summarized in Table 1.
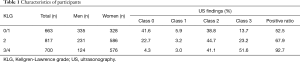
Full table
Clinical manifestation
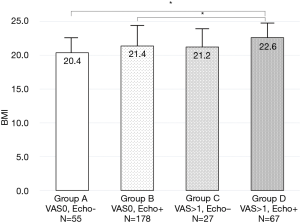
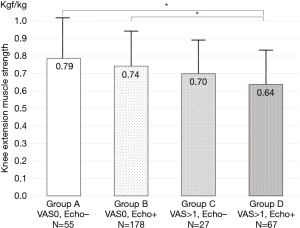
To clarify clinical characteristics of early knee OA, 663 knees with KLG 0 and 1 were divided into four groups in terms of pain and US findings; Group A (with no pain and echo-negative findings, n=192), Group B (with no pain and echo-positive findings, n=284), Group C (with pain and echo-negative findings, n=84), and Group D (with pain and echo-positive findings, n=103).
VAS of pain was significantly different among the groups (Table 2). There were no significant differences in BMI among the groups in men, however, BMI was significantly increased with pain and US findings in women (Figure 2).
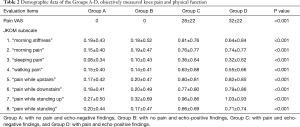
Full table
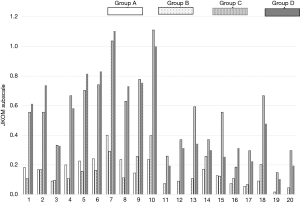
Group C and D indicated a significant higher JKOM score than Group A and B, especially in terms of the disturbance of ADLs such as ascending upstairs or descending downstairs, standing up, and squatting. Subtotal JKOM score of ADLs, social activities and general health conditions except pain and stiffness was increased with pain and US findings (Tables 2,3 & Figure 3). Group B showed significant higher subscales** in the disturbance of those ADLs than Group A, although Group B has no pain (Figure 3).
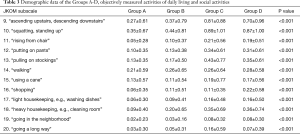
Full table
Muscle strength
Maximum knee extension muscle strength in Group C and D were weaker those in Group A and B. Group D indicated the weakest muscle strength among the groups. The muscle strength weakness in women was significantly correlated with pain (Figure 4).
Risk factors
Adjusted odds ratios of proceeding Group D from Group B in women, high BMI (≥25), and muscle weakness (< average knee extension muscle strength) were 1.30 (95% CI: 0.80–2.14, P=0.290), 1.86 (95% CI: 1.04–3.33, P=0.034), and 1.19 (95% CI: 0.74–1.91, P=0.162). Adjusted odds ratios of proceeding Group B from Group A in women, high BMI (≥25), and muscle weakness (< average knee extension muscle strength) were 1.30 (95% CI: 0.80–2.14, P=0.290), 1.86 (95% CI: 1.04–3.33, P=0.034), and 1.19 (95% CI: 0.74–1.91, P=0.162).
Discussion
In a large residential health examination of Japanese with confirmed early KOA by US and radiographs, ADLs such as ascending upstairs or descending downstairs, standing up, and squatting were most likely to be the first disturbances with pain. This study also demonstrates that painful knees indeed disturbed these ADLs, however did so even pain-free knees with US findings. This means that pain was not always necessary to disturb these ADLs. Women, high BMI, and weak knee extension muscle strength except pain might be contributed to the disturbances. Weight-bearing bending of the knee in these ADLs needs muscle power to maintain the posture and physical activity. Women who have weak knee extension muscle strength, high BMI which cause over load stress to the knee joint, and weak knee extension muscle strength are the risk factors proceeding to early KOA. Therefore, early detection of the disturbances of these ADLs might benefit care management of high-risk members in a community, monitoring for their process and prevention of established KOA.
To our knowledge it is the first study to determine which activities might be associated with pain of early KOA. According to Madry et al. (23), one of the first symptoms is pain while climbing stairs and the severity of pain is increased while kneeling or squatting activities. However, they did not show clinical evidences referred to these findings. It might be theoretically right that activities to bend the knee joint with weight seem to cause initial pain following development of KOA, but this might have not been proven. Hensor et al. assessed the WOMAC data of 4673 people obtained from the Osteoarthritis Initiative (OAI), determining which activities might be related to the start of knee pain (24). With Rasch analysis to rank the WOMAC pain questions (activities), they concluded that knee pain would appear first during weight-bearing activities regarding bending of the knee, such as using stairs. Our results that ADLs such as ascending upstairs or descending downstairs, standing up, and squatting were disturbed even in early KOA, were well corresponded to those of Hensor et al. (24). Moreover, pain-free knees with OA positive US finding also disturbed these ADLs, which might be not caused by pain but weak knee extension muscle strength.
From a practical point of view, Nguyen et al. (25) reviewed and summarized that strengthening the quadriceps may improve joint stability (26), and that optional strengthening the quadriceps and medial or lateral muscle to decrease the load in the medial compartment (27) or the lateral patellofemoral compartment. The OA Research Society International (OARSI) recommendations in 2014 (28) said that muscle training was even regarded as a core treatment of KOA and is proposed for all patients. Brosseau et al. reviewed 26 high-quality studies and showed that strengthening exercise programs significantly improved pain relief, physical function and quality of life (29). Strengthening knee extension muscle might not only provide pain relief and functional improvement of established KOA, but also prevent from proceeding pre-stage of early KOA to the early or established one.
There are some restrictions to the current study. This is a cross-sectional study using participants recruited at residential health examinations, not a longitudinal cohort study. It is impossible to accurately predict the onset of early KOA from pain-free knee, but the present study might indicate some risk factors such as woman, high BMI, and weak knee extension muscle strength. Another restriction is the usage of US to detect early KOA, instead of arthroscopy and/or MRI. Arthroscopy and MRI would confirm structural deterioration of KOA which is not apparent with standard radiographs. For residential health examinations, however, US is a fast, easy-to-use, safe, and low cost for public health service.
In conclusion, symptoms of early KOA might be not only pain but also the disturbance of ADLs such as ascending upstairs or descending downstairs, standing up, and squatting with weak knee extension muscle strength (as the physical finding of early KOA). Even pain-free knees with positive US findings when disturbing those ADLs, might be the pre-stage of early KOA, namely super-early KOA. For prevention of proceeding to early KOA, strengthening of the knee extension muscle might be important to maintain these ADLs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Early Osteoarthritis: Definition, Pathogenesis, Diagnosis, Management and Prevention”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.09.06). The series “Early Osteoarthritis: Definition, Pathogenesis, Diagnosis, Management and Prevention” was commissioned by the editorial office without any funding or sponsorship. YU served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Shimane University School of Medicine approved the present study, and all participants provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hunter W. Of the structure and disease of articulating cartilages. 1743. Clin Orthop Relat Res 1995;3-6. [PubMed]
- Centers for Disease Control and Prevention (CDC). Prevalence of doctor diagnosed arthritis and arthritis attributable activity limitation—United States, 2007-2009. MMWR Morb Mortal Wkly Rep 2010;59:1261-5. [PubMed]
- D’Ambrosia RD. Epidemiology of osteoarthritis. Orthopedics 2005;28:s201-5. [PubMed]
- Felson DT, Naimark A, Anderson J, et al. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum 1987;30:914-8. [Crossref] [PubMed]
- Yoshimura N, Muraki S, Oka H, et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab 2009;27:620-8. [Crossref] [PubMed]
- Xie F, Kovic B, Jin X, et al. Economic and Humanistic Burden of Osteoarthritis: A Systematic Review of Large Sample Studies. Pharmacoeconomics 2016;34:1087-100. [Crossref] [PubMed]
- Murphy L and Helmick CG. The Impact of Osteoarthritis in the United States: A Population- Health Perspective. Available online: https://www.usbji.org/sites/default/files/ COAMI%20Impact%20of%20OA% 20in%20the%20US.pdf (accessed September 6, 2018).
- World Health Organization. 10 facts on ageing and health. Available online: http://www.who.int/features/factfiles/ageing/en/ (accessed September 6, 2018).
- Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039-49. [Crossref] [PubMed]
- Menkes CJ. Radiographic criteria for classification of osteoarthritis. J Rheumatol Suppl 1991;27:13-5. [PubMed]
- Keuttner K, Goldberg VM: Osteoarthrtic Disorders. Rosemont: American Academy of Orthopaedic Surgeons, pp xxi-v.
- Luyten FP, Denti M, Filardo G, et al. Definition and classification of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 2012;20:401-6. [Crossref] [PubMed]
- International Cartilage Regeneration and Joint Preservation Society. ICRS Clinical Cartilage Injury Evaluation System. Available online: https://cartilage.org/society/publications/icrs-score/?highlighted= ICRS %20score (accessed September 6, 2018).
- Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12:177-90. [Crossref] [PubMed]
- Hunter DJ, Lo GH, Gale D, et al. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score). Ann Rheum Dis 2008;67:206-11. [Crossref] [PubMed]
- Akai M, Doi T, Fujino K, et al. An outcome measure for Japanese people with knee osteoarthritis. J Rheumatol 2005;32:1524-32. [PubMed]
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494-502. [Crossref] [PubMed]
- Saarakkala S, Waris P, Waris V, et al. Diagnostic performance of knee ultrasonography for detecting degenerative changes of articular cartilage. Osteoarthritis Cartilage 2012;20:376-81. [Crossref] [PubMed]
- Lee CL, Huang MH, Chai CY, et al. The validity of in vivo ultrasonographic grading of osteoarthritic femoral condylar cartilage: a comparison with in vitro ultrasonographic and histologic gradings. Osteoarthritis Cartilage 2008;16:352-8. [Crossref] [PubMed]
- Yoon CH, Kim HS, Ju JH, et al. Validity of the sonographic longitudinal sagittal image for assessment of the cartilage thickness in the knee osteoarthritis. Clin Rheumatol 2008;27:1507-16. [Crossref] [PubMed]
- Aula AS, Töyräs J, Tiitu V, et al. Simultaneous ultrasound measurement of articular cartilage and subchondral bone. Osteoarthritis Cartilage 2010;18:1570-6. [Crossref] [PubMed]
- Narumi K, Funaki Y, Yoshimura N, et al. Quadriceps muscle strength reference value as index for functional deterioration of locomotive organs: Data from 3617 men and women in Japan. J Orthop Sci 2017;22:765-70. [Crossref] [PubMed]
- Madry H, Kon E, Condello V, et al. Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 2016;24:1753-62. [Crossref] [PubMed]
- Hensor EM, Dube B, Kingsbury SR, et al. Toward a clinical definition of early osteoarthritis: onset of patient-reported knee pain begins on stairs. Data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2015;67:40-7. [Crossref] [PubMed]
- Nguyen C, Lefèvre-Colau MM, Poiraudeau S, et al. Rehabilitation (exercise and strength training) and osteoarthritis: A critical narrative review. Ann Phys Rehabil Med 2016;59:190-5. [Crossref] [PubMed]
- Felson DT. Clinical Practice. Osteoarthritis of the knee. N Engl J Med 2006;354:841-8. [Crossref] [PubMed]
- Rannou F, Poiraudeau S. Non-pharmacological approaches for the treatment of osteoarthritis. Best Pract Res Clin Rheumatol 2010;24:93-106. [Crossref] [PubMed]
- McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363-88. [Crossref] [PubMed]
- Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil 2017;31:596-611. [Crossref] [PubMed]
Cite this article as: Uchio Y, Takuwa H, Kuwata S, Kumahashi N. Symptoms and physical findings of early knee osteoarthritis. Ann Joint 2018;3:79.