
“Conservative” surgical treatment for anterior knee pain
A comprehensive understanding of the role of surgery in the treatment of anterior knee pain (AKP) requires understanding the concept of homeostasis and accepting the hypothesis that significant loss of tissue homeostasis results in pain (1). Some tissue is under excessive load because there has been an acute injury or chronic overload. When challenged by overload of an acute or chronic nature, our musculoskeletal tissues demonstrate a remarkable ability to heal (return to asymptomatic homeostasis) given appropriate loading conditions and enough time.
Loss of homeostasis provokes pain by multiple physiological pathways. Soft tissue pain can result from chronic nerve injury, neural growth factor, ischemia, prostaglandins, and substance P (2-4). In addition, bone pain can result from osseous hypertension and venous congestion (5-6). AKP can range from incapacitating to merely annoying. Since it is ultimately the severity of the perceived pain that determines the extent of treatment, we must strive to understand well not only the purely biological pathology but also the psychological factors contributing to the perception of pain.
The perception of painful impulses varies from patient to patient. Sanchis-Alfonso and others have studied these factors in the evaluation and treatment of AKP (7-10). In the overall mosaic of factors that create or disturb clinical homeostasis, mental factors must not be neglected. In a sense, psychological factors such as kinesiophobia and catastrophization affect mental “homeostasis” and must be considered in treatment. Depression and anxiety may also co-exist in patients with AKP. But we must remember that in most cases such conditions affect the perception of pain but are not the cause of the pain. Doménech et al. and Piva et al. have shown that psychological characteristics such as catastrophizing, anxiety, depression and kinesiophobia were reduced after treatment of AKP (7,9). It is important to remember these psychological conditions may coexist with AKP. This study is an encouragement to providers of the positive mental effects of successful treatment of AKP.
Other psychological conditions such as somatic symptom disorder (previously called somatization disorder) may represent unusual situations where the cause of pain could be a result of the actual psychological condition. Somatization is the experience and expression of psychological distress through bodily symptoms (11). Such situations of somatization are clearly diagnoses of exclusion and benefit from meticulous clinical evaluation to exclude anatomic/physiological conditions and must be treated in consultation with mental health professionals familiar with these conditions.
It would be nice to be able to say that patient and thorough non-operative treatment can always result in homeostasis and pain relief but it does not. Even when principles of relative rest, judicious pharmacologic treatment, flexibility and strength restoration, whole extremity/whole person rehab and living within the envelope of function are employed, symptoms can remain. In such cases it is imperative to have a conservative and precise approach to surgical options.
So, what is “conservative” surgical treatment?
The term “conservative” has been used so often in the context of non-operative care that it has sadly almost become synonymous with non-surgical care. But the Merriam Webster dictionary definition of conservative (the non-political definition) is “marked by moderation or caution” (12). This is the key to a proper surgical approach. Certainly “conservative” should not apply just to non-operative care. When needed, we must do just enough surgery to provide a proper stimulus to allow natural healing to restore homeostasis. Less is better. Avoid excess.
Make the right diagnosis
A specific diagnosis is paramount. This can result only from careful history and physical examination and wise use of imaging. As this topic has been comprehensively covered in a previous chapter, I will simply highlight the factors from clinical evaluation that help make specific diagnoses that may respond to surgery.
There are several key questions to answer:
- Is the pain source intra-articular or extra-articular?
- Is there a structurally deficient articular surface?
- Is there objective “malalignment” causing overload?
Careful palpation is critical to properly diagnose and direct treatment. Focal tenderness is an important piece of the diagnostic puzzle. Simple pain diagrams have been proven to correlate with tenderness and are an inexpensive helpful tool (13). Prior scars are potential neuroma locations. Plica tenderness when prominent is an easy diagnosis. Tenderness over the quadriceps or patellar tendon attachments to the patella strongly suggest focal intra-tendon pathology and surrounding inflammation. Tinel’s sign over the infra-patellar branch of the saphenous nerve is another important finding that can direct treatment. Tenderness of the infrapatellar fat pad is an important finding as is Hoffa’s sign.
If there is a palpable joint effusion present, this represents evidence of some degree of intra-articular inflammation. It is not the effusion that is the actual problem but “where there is smoke, there is fire” and the presence of an effusion should stimulate careful search for intra-articular pathology. Chondral, or synovial pathology can produce effusions. Do not forget about inflammatory arthritis in the differential diagnosis.
Intra-articular injection can be useful both diagnostically and therapeutically. In the absence of medical contra-indications, injection with 10 cc of bupivacaine combined with a local acting steroid preparation should provide some acute pain relief if there is a significant component of an intra-articular pain source. When I do this, the patient remains in the office for at least 15 minutes and is re-examined for objective (gait, squatting, resolution of tenderness) and subjective relief. Direct injection of symptomatic plica can be very useful on occasion (14). One must remember that there could be some placebo effect to any injection, but response to injection is another important clue.
Similarly, extra-articular injection with a smaller volume of local anesthetic can serve the same purpose when a focal area of soft tissue tenderness is discovered. This can be useful particularly when a neuroma is suspected or in the presence of other focal retinacular tenderness (15).
Imaging is yet another piece of the diagnostic puzzle. X-ray and/or CT scan provide key information about bone lesions, alignment, joint space narrowing, osteophytes and possible loose bodies. Bone scan is a sensitive way of assessing the degree of current biological response to injury or overuse. A single-photon emission computerized tomography (SPECT) scan can be a tremendous help in sorting out complex cases since bone and alignment are precisely seen plus an assessment of the areas of biological overload or injury. In situations where surgical unloading is to be considered, I have felt that a biological overload assessment (bone scan or SPECT) is prudent to be sure of the surgical plan. MRI scan can provide much of this information but scans are often of variable quality and image sequences inconsistent to provide consistent evaluation of focal overload. Ultrasound is a useful and inexpensive way to evaluate focal areas of suspected tendinosis.
Before we discuss specific surgical interventions, we must pause and consider the question of patellofemoral malalignment. “Malalignment” has been often discussed as a major factor causing AKP and has been extensively studied and recommendations for surgical treatment have often centered on searching for and correcting perceived malalignment. One problem with this approach is that anatomic malalignment as often defined by imaging measurements is commonly seen in asymptomatic patients. We must look beyond the imaging study to understand malalignment (16). Incorporating Dye’s principles of homeostasis and envelope of function it is perhaps better to define patellofemoral “alignment” as the result of a combination of passive (soft tissue, bony) constraints and dynamic factors (strength/flexibility/neuro-muscular control) (16).
Using this expanded definition of alignment, malalignment then can be defined to be when bony alignment, joint geometry, soft tissue restraints, neuromuscular control and functional demands combine to produce symptoms as a result of abnormally directed loads which exceed the physiological threshold of the tissues. Clearly all these factors cannot be discerned on a viewbox. Careful clinical evaluation is paramount (16). No single imaging finding defines absolute indication for a surgery or predicts failure of non-op treatment.
How can surgery help?
Removing the focus of inflammatory soft tissue pain
When a focal soft tissue source of pain can be identified such as a pathologic hypertrophic medial parapatellar plica, fat pad hypertrophy and scarring, or localized synovial hypertrophy debridement of the pathologic tissue can provide dramatic relief.
As has been shown by Dorchak et al., pre-operative diagnosis of the pathologic medial parapatellar plica and arthroscopic findings of chondral abrasion from the plica impingement correlates with better results after plica excision (17).
Focal areas of hypertrophic synovitis sometimes cause AKP and fail to respond to non-operative treatment. In such cases, arthroscopy must be done very carefully to find focal area of synovial hypertrophy which are often peri-patellar, most common medially and at the inferior pole of the patella in my experience. Meticulous debridement of focal areas of synovitis that correlate with symptoms is a valuable and minimally invasive treatment (Figure 1). During arthroscopy, one must look deliberately at all the synovial surfaces, plicae (if present) and the fat pad. Use of a proximal medial arthroscopic portal has been very helpful in some complex cases of anterior compartment soft tissue pathology (18). Looking from this portal provides a panoramic view of the anterior soft tissues and avoids the potential error of looking past the pathology when inserting the scope from a peri-patellar tendon portal.
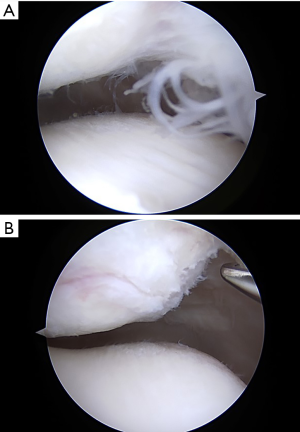
The fat pad is among the most densely innervated tissues in the knee (19). In vivo arthroscopic palpation of the un-anesthetized fat pad produces severe pain (20). When the fat pad mobility is limited or pathologically hypertrophied and impinged upon by surrounding structures, pain results. This pain often responds to non-operative measures but may require surgery. Arthroscopic treatment should include meticulous resection of the hypertrophied and impinging fat pad and surrounding synovitis (21).
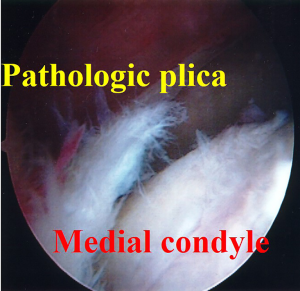
Synovial plicae are normal structures that can become pathologic when thickened and inflamed. The medial plica is the most commonly symptomatic and can be palpated on physical exam. Focal tenderness reproducing symptoms makes this an easy diagnosis. When unresponsive to non-surgical care, excision of the plica and surrounding synovitis is frequently curative (22,23). Such truly pathologic plicae when chronic have often produced areas of chondral change along the medial trochlea from chronic impingement (Figure 2). When resection such a plica it is important to remove not only the synovitis but the entire band thickened plical tissue down to the medial plica. Intra-operative range of motion (ROM) should confirm no further soft tissue impingement.
When there is focal scarring post-operatively of the anterior interval as defined by Steadman after injury or surgery, AKP may result (24). Cadaveric study also documents biomechanical changes and patella infera with such simulated adhesion (25). Specifically, this involves scarring in the interval between the posterior fat pad and the proximal tibia just posterior to it as well as to the intermeniscal ligament. These tissues are normally mobile and during arthroscopy in a normal knee one can see anterior movement of the fat pad relative to the menisci and intermeniscal ligament with knee extension. Arthroscopic release of this contracture produced good results likely from relief of pathologic tension upon the very highly innervated fat pad.
With all these arthroscopic procedures, careful hemostasis and gentle post-operative rehabilitation is essential. Avoiding postoperative effusion or hemarthrosis greatly facilitates recovery as joint effusions as small as 30cc have been shown to produce arthrogenic quadriceps muscle inhibition experimentally (26,27).
Stimulating a healing environment
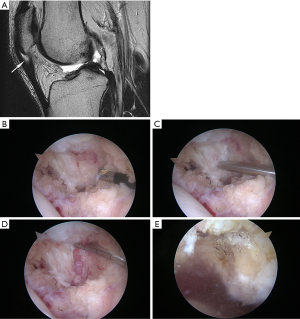
When injury or overuse have resulted in an area of localized tendinosis, non-operative treatment almost always works. It has been well documented in the literature that tendinosis can be asymptomatic even in elite athletes (28,29) so it is critical that this diagnosis be made primarily clinically with imaging as confirmation when necessary. Treatments including stretching, gentle eccentric exercise within the envelope of function, ultrasound-guided platelet-rich plasma (PRP) injection and extracorporeal shock wave have support in the literature (30-32). Surgery to debride the pathologic tissue can be very effective in the unusual situation where non-operative treatment fails. The principle here is just as with other surgeries for tendinosis such as lateral elbow tendinosis. Surgical excision of the pathologic tissue provides a stimulus for healing tissue, which exposed then to proper loads can produce healing and restoration of function. Procedures described often include abrasion of the inferior pole of the patella as well. Arthroscopic and open procedures have been described. While the extent of debridement has varied among various open techniques (33,34) good results have been reported. Likewise, arthroscopic techniques have been described with similar success (26,35). Figure 3 shows MRI of typical patellar tendinosis and surgical findings during debridement. Of interest is that when the procedure is done arthroscopically one consistent finding is focal synovitis at the posterior surface of the patellar tendon near the pathologic tendinosis. In fact, one study focused on debridement of this inflammatory focus behind the tendon without tendon debridement and had very good results (37). This area of synovitis and fat pad hypertrophy is also accompanied by hypervascularity on the posterior proximal tendon. The ability to precisely address this area of inflammatory soft tissue hypertrophy may be an advantage at least theoretically of the arthroscopic approach, though trans-tendinous open surgery could potentially reach the same tissue. This is not an operation that should be needed very often. Soft tissue pain can also come from retinacular structures. Focal findings in retinacular tissues that are unresponsive to non-operative care are unusual but can be successfully treated by local debridement of the retinaculum (15). The key to finding such sources of soft tissue pain is careful palpation.
Releasing pathologically tight structures
Lateral retinacular release (LRR)was originally introduced as procedure for recurrent patellar instability and AKP. Long-term follow up has shown LRR for instability to have a high recurrence rate and LRR has been abandoned as a treatment for patellofemoral instability (38-40). When patients have AKP, hypomobile tender lateral retinaculum, excessive lateral patellar tilt (without lateral translation) on imaging study and minimal articular degeneration (Outerbridge I/II) LRR can be considered (41). Lateral release can be done arthroscopically or by an open procedure. In either case, one must avoid damaging the vastus lateralis tendon proximally. Iatrogenic medial patellar instability is a disabling complication of lateral release and can occur after excessive lateral release or in the setting of lateral release done in the absence of true lateral pathologic hypomobility (42-44). Lateral retinacular lengthening is an alternative procedure that has demonstrated equivalent efficacy with a lower rate of iatrogenic medial instability (45,46). Lateral retinacular lengthening is done as an open procedure. The superficial layer of the lateral retinaculum is incised along the length of the patella. Dissection then proceeds posteriorly between the superficial and deep layers. The deep layer is then transected longitudinally 2 cm posterior to the initial incision in the superficial retinaculum. Any soft tissue tethers distal to the deep layer of the retinaculum also should be released to allow patellar tilt to be unconstrained by any soft tissue. The proximal extent of the release should be at least to the superior pole of the patella. If release must extend more proximally than this, one must be certain to protect the vastus lateralis tendon insertion. The anterior edge of the deep retinacular layer is then sutured to the posterior limb of the superficial retinaculum without tension with the knee flexed. The knee should be carefully tested though full ROM to be sure that the lengthening repair is secure. Careful attention to hemostasis is needed as the superior lateral geniculate vessels are a common cause of hemarthrosis. If the procedure is done under tourniquet, the tourniquet should be release and hemostasis assured prior to wound closure. In actual practice lateral release or lengthening is very rarely needed as an isolated procedure given the success of contemporary non-operative management is such patients. In a survey of the International Patellofemoral Study Group, a group with special expertise and experience in treatment of patellofemoral disorders, lateral release was rarely performed (40). In my opinion, the best way to avoid complications if surgery is absolutely needed for AKP in this clinical scenario is to consider a lateral lengthening procedure. That said, in my personal experience in a busy sports medicine practice, it has been years since I have needed to recommend isolated LRR or lateral lengthening.
Relieving articular overload
In the presence of pathologic lateral tilt/lateral translation with Outerbridge III–IV changes, and minimal tibiofemoral arthrosis, anteromedialization (AMZ) of the tibial tuberosity as described by Fulkerson is very effective (47-49). Distal and lateral articular lesions respond best to this unloading osteotomy (50). While this is a major open procedure, pain relief is reproducible. Results are consistently good and patients satisfied but this procedure does not result in normal knee function when there are Outerbridge III–IV changes (48,51-53). Consequently, AMZ has been combined with attempts at biological resurfacing in these challenging patients with encouraging results (54-56). The exact indications for when complex biological resurfacing protocols are indicated are still evolving.
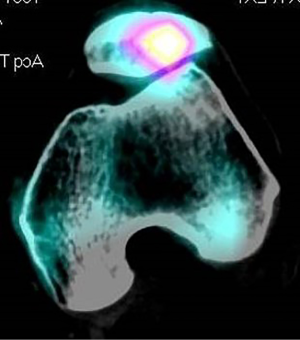
When considering anteromedialization, it seems prudent to require clear imaging evidence of lateral patellar facet overload. In my practice I prefer SPECT scan for this purpose since it provides precise alignment data as well as biological data from the nuclear part of the study (Figure 4). High quality MRI scan or bone scans may give similar information. If such advanced studies are not available, patellar axial views in early flexion should show evidence of lateral facet sclerosis and joint space narrowing.
Various manufacturers have produced instrumentation for AMZ but specific guides are not necessarily required as basic orthopedic instrumentation suffices. Fulkerson’s original technique has been reproducible for me (47). Arthroscopy is always done at the time of AMZ to search for other intra-articular pathology and to debride focal areas of synovitis and unstable chondral fragments. Biological resurfacing which might include abrasion/microfracture/autologous chondrocyte implantation are considerations, but in my practice I have been very satisfied with minimalist debridement of unstable fragments. Important surgical pearls include fully mobilizing any adhesions between the patellar tendon and the fat pad, performing adequate lateral release sparing the vastus lateralis, being certain to make the osteotomy steep enough to accomplish anteriorization in the 15-mm range, using bicortical lag screw fixation, countersinking the heads of the fixation screws and assuring meticulous hemostasis. Tapering the osteotomy distally to avoid notching of the anterior tibial cortex is essential. Early quadriceps exercises, ROM as tolerated and partial weight bearing are key for the first 6 weeks.
Case reports of tibial fracture with early full weight bearing have modified early recommendations for more aggressive rehabilitation (57,58). This is a major open operation and there is no reason to rush aggressive rehabilitation. Tibial fractures have reported as late as 3–6 months post-operatively (59). Avoidance of notching the anterior tibial cortex was emphasized by multiple authors to prevent this complication (58,59). The knee has lots of healing to do toward restoring ultimate homeostasis and rehab must be patient. Improvements in function typically continue for six months or more in these patients, though many are improved compared to pre-operative status by three months.
Improve the surfaces by biological or prosthetic resurfacing
Prominent focal chondral flaps may be the source of notable AKP when such unstable fragments are present on the patella or trochlea. In such cases I prefer a minimalist debridement of only unstable fragments. More aggressive chondral procedures can be done as secondary procedures if needed and often no further surgery is needed. As noted above in the discussion regarding anteromedialization, biological resurfacing procedures for focal chondral defects have become more successful when combined with appropriate unloading and realignment procedures. In extreme situations, unipolar or even bipolar osteochondral allografts have been used in young patients. Results have been encouraging in small series and techniques continue to evolve (60,61). In severe cases of malalignment and arthrosis, patellofemoral arthroplasty (PFA) can be very successful (Figure 5). Just as any other prosthetic arthroplasty careful consideration of a patient’s physiological age and activity demands are key to the longevity of the treatment. Return to a patient’s desired activity level has recently been reported to occur in 72% of patients (62). Generally, such patients are severely disabled before surgical treatment and may experience dramatic improvement postoperatively. Early experience with PFA was complicated by alignment, instability and implant issues but as surgical techniques and implants have improved, so have results. Nonetheless, PFA like other infrequently needed procedures for AKP should be done primarily by surgeons well trained and confident in handling complex patellofemoral pathology.
Before closing this article, I hasten to say, surgery for AKP is a last resort and not very often needed. But when focal pathology can be identified logical intervention can be very successful. Minimalist approach to debridement of inflammatory foci of synovium and fat pat, conservative debridement of unstable chondral fragments can bring dramatic improvements. Sometimes major surgery such as AMZ and PFA is necessary, but remember there are no absolute radiographic indications for such major surgery and patients with notable imaging pathology are commonly improved without surgery. Gentle rehabilitation within the patient’s envelope of function must always be the rule. Restoration of tissue homeostasis by these techniques can make a tremendous difference in these patients’ lives. Likewise, poorly executed or ill-advised surgical intervention can be catastrophic. Careful clinical judgement is paramount. Any surgical procedure should be “conservative”—marked by moderation and caution. Primum non nocere.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vicente Sanchis-Alfonso and Scott F. Dye) for the series “The Patellofemoral Joint” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.08.01). The series “The Patellofemoral Joint” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Post WR, Dye SF. Patellofemoral Pain: An Enigma Explained by Homeostasis and Common Sense. Am J Orthop (Belle Mead NJ) 2017;46:92-100. [PubMed]
- Fulkerson JP, Tennant R, Jaivin JS, et al. Histologic evidence of retinacular nerve injury associated with patellofemoral malalignment. Clin Orthop Relat Res 1985;196-205. [PubMed]
- Sanchis-Alfonso V, Roselló-Sastre E, Monteagudo-Castro C, et al. Quantitative analysis of nerve changes in the lateral retinaculum in patients with isolated symptomatic patellofemoral malalignment. A preliminary study. Am J Sports Med 1998;26:703-9. [Crossref] [PubMed]
- Sanchis-Alfonso V, Roselló-Sastre E, Revert F, et al. Histologic retinacular changes associated with ischemia in painful patellofemoral malalignment. Orthopedics 2005;28:593-9. [PubMed]
- Dye SF, Boll DA. Radionuclide imaging of the patellofemoral joint in young adults with anterior knee pain. Orthop Clin North Am 1986;17:249-62. [PubMed]
- Waisbrod H, Treiman N. Intra-osseous venography in patellofemoral disorders. A preliminary report. J Bone Joint Surg Br 1980;62-B:454-6. [Crossref] [PubMed]
- Piva SR, Fitzgerald GK, Wisniewski S, et al. Predictors of pain and function outcome after rehabilitation in patients with patellofemoral pain syndrome. J Rehabil Med 2009;41:604-12. [Crossref] [PubMed]
- Thomeé P, Thomee R, Karlsson J. Patellofemoral pain syndrome: pain, coping strategies and degree of well-being. Scand J Med Sci Sports 2002;12:276-81. [Crossref] [PubMed]
- Doménech J, Sanchis-Alfonso V, Espejo B. Changes in catastrophizing and kinesiophobia are predictive of changes in disability and pain after treatment in patients with anterior knee pain. Knee Surg Sports Traumatol Arthrosc 2014;22:2295-300. [Crossref] [PubMed]
- Domenech J, Sanchis-Alfonso V, López L, et al. Influence of kinesiophobia and catastrophizing on pain and disability in anterior knee pain patients. Knee Surg Sports Traumatol Arthrosc 2013;21:1562-8. [Crossref] [PubMed]
- Busch FN. Clinical approaches to somatization. J Clin Psychol 2014;70:419-27. [Crossref] [PubMed]
-
Dictionary M.-W.O. - Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy 1994;10:618-23. [Crossref] [PubMed]
- Rovere GD, Adair DM. Medial synovial shelf plica syndrome. Treatment by intraplical steroid injection. Am J Sports Med 1985;13:382-6. [Crossref] [PubMed]
- Kasim N, Fulkerson JP. Resection of clinically localized segments of painful retinaculum in the treatment of selected patients with anterior knee pain. Am J Sports Med 2000;28:811-4. [Crossref] [PubMed]
- Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med 2002;21:521-46. x. [Crossref] [PubMed]
- Dorchak JD, Barrack RL, Kneisl JS, et al. Arthroscopic treatment of symptomatic synovial plica of the knee. Long-term followup. Am J Sports Med 1991;19:503-7. [Crossref] [PubMed]
- Schreiber SN. Proximal superomedial portal in arthroscopy of the knee. Arthroscopy 1991;7:246-51. [Crossref] [PubMed]
- Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med 1982;10:329-35. [Crossref] [PubMed]
- Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med 1998;26:773-7. [Crossref] [PubMed]
- Dragoo JL, Johnson C, McConnell J. Evaluation and treatment of disorders of the infrapatellar fat pad. Sports Med 2012;42:51-67. [Crossref] [PubMed]
- Yilmaz C, Golpinar A, Vurucu A, et al. Retinacular band excision improves outcome in treatment of plica syndrome. Int Orthop 2005;29:291-5. [Crossref] [PubMed]
- Flanagan JP, Trakru S, Meyer M, et al. Arthroscopic excision of symptomatic medial plica. A study of 118 knees with 1-4 year follow-up. Acta Orthop Scand 1994;65:408-11. [Crossref] [PubMed]
- Steadman JR, Dragoo JL, Hines SL, et al. Arthroscopic release for symptomatic scarring of the anterior interval of the knee. Am J Sports Med 2008;36:1763-9. [Crossref] [PubMed]
- Ahmad CS, Kwak SD, Ateshian GA, et al. Effects of patellar tendon adhesion to the anterior tibia on knee mechanics. Am J Sports Med 1998;26:715-24. [Crossref] [PubMed]
- Spencer JD, Hayes KC, Alexander IJ. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil 1984;65:171-7. [PubMed]
- Palmieri-Smith RM, Kreinbrink J, Ashton-Miller JA, et al. Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med 2007;35:1269-75. [Crossref] [PubMed]
- Cook JL, Khan KM, Kiss ZS, et al. Asymptomatic hypoechoic regions on patellar tendon ultrasound: A 4-year clinical and ultrasound followup of 46 tendons. Scand J Med Sci Sports 2001;11:321-7. [Crossref] [PubMed]
- Cook JL, Khan KM, Kiss ZS, et al. Prospective imaging study of asymptomatic patellar tendinopathy in elite junior basketball players. J Ultrasound Med 2000;19:473-9. [Crossref] [PubMed]
- Liddle AD, Rodriguez-Merchan EC. Platelet-Rich Plasma in the Treatment of Patellar Tendinopathy: A Systematic Review. Am J Sports Med 2015;43:2583-90. [Crossref] [PubMed]
- Everhart JS, Cole D, Sojka JH, et al. Treatment Options for Patellar Tendinopathy: A Systematic Review. Arthroscopy 2017;33:861-72. [Crossref] [PubMed]
- Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med 2014;42:610-8. [Crossref] [PubMed]
- Shelbourne KD, Henne TD, Gray T. Recalcitrant patellar tendinosis in elite athletes: surgical treatment in conjunction with aggressive postoperative rehabilitation. Am J Sports Med 2006;34:1141-6. [Crossref] [PubMed]
- Popp JE, Yu JS, Kaeding CC. Recalcitrant patellar tendinitis. Magnetic resonance imaging, histologic evaluation, and surgical treatment. Am J Sports Med 1997;25:218-22. [Crossref] [PubMed]
- Pascarella A, Alam M, Pascarella F, et al. Arthroscopic management of chronic patellar tendinopathy. Am J Sports Med 2011;39:1975-83. [Crossref] [PubMed]
- Romeo AA, Larson RV. Arthroscopic treatment of infrapatellar tendonitis. Arthroscopy 1999;15:341-5. [Crossref] [PubMed]
- Maier D, Bornebusch L, Salzmann GM, et al. Mid- and long-term efficacy of the arthroscopic patellar release for treatment of patellar tendinopathy unresponsive to nonoperative management. Arthroscopy 2013;29:1338-45. [Crossref] [PubMed]
- Post WR, Fithian DC. Patellofemoral Instability: A Consensus Statement From the AOSSM/PFF Patellofemoral Instability Workshop. Orthop J Sports Med 2018;6:2325967117750352 [Crossref] [PubMed]
- Liu JN, Steinhaus ME, Kalbian IL, et al. Patellar Instability Management: A Survey of the International Patellofemoral Study Group. Am J Sports Med 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Fithian DC, Paxton EW, Post WR, et al. Lateral retinacular release: a survey of the International Patellofemoral Study Group. Arthroscopy 2004;20:463-8. [Crossref] [PubMed]
- Ford DH, Post WR. Open or arthroscopic lateral release. Indications, techniques, and rehabilitation. Clin Sports Med 1997;16:29-49. [Crossref] [PubMed]
- Sanchis-Alfonso V, Merchant AC. Iatrogenic Medial Patellar Instability: An Avoidable Injury. Arthroscopy 2015;31:1628-32. [Crossref] [PubMed]
- Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med 1988;16:383-8. [Crossref] [PubMed]
- Teitge RA, Torga Spak R. Lateral patellofemoral ligament reconstruction. Arthroscopy 2004;20:998-1002. [Crossref] [PubMed]
- Pagenstert G, Wolf N, Bachmann M, et al. Open lateral patellar retinacular lengthening versus open retinacular release in lateral patellar hypercompression syndrome: a prospective double-blinded comparative study on complications and outcome. Arthroscopy 2012;28:788-97. [Crossref] [PubMed]
- O'Neill DB. Open lateral retinacular lengthening compared with arthroscopic release. A prospective, randomized outcome study. J Bone Joint Surg Am 1997;79:1759-69. [Crossref] [PubMed]
- Post WR, Fulkerson JP. Distal realignment of the patellofemoral joint. Indications, effects, results, and recommendations. Orthop Clin North Am 1992;23:631-43. [PubMed]
- Fulkerson JP, Becker GJ, Meaney JA, et al. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med 1990;18:490-6; discussion 496-7. [Crossref] [PubMed]
- Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop 1983;176-81. [PubMed]
- Pidoriano AJ, Weinstein RN, Buuck DA, et al. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med 1997;25:533-7. [Crossref] [PubMed]
- Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res 1990;242-50. [PubMed]
- Bellemans J, Cauwenberghs F, Witvrouw E, et al. Anteromedial tibial tubercle transfer in patients with chronic anterior knee pain and a subluxation-type patellar malalignment. Am J Sports Med 1997;25:375-81. [Crossref] [PubMed]
- Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg 2008;21:101-5. [Crossref] [PubMed]
- Herring MJ, Rud CT, Macalena JA. Autologous Chondrocyte Implantation Using a Bilayer Collagen Membrane with Bone Graft and Anteromedialization of the Tibial Tubercle for the Treatment of a Large Osteochondral Defect in the Lateral Knee Trochlea: A Case Report. JBJS Case Connect 2016;6:e35 [Crossref] [PubMed]
- Gillogly SD, Arnold RM. Autologous chondrocyte implantation and anteromedialization for isolated patellar articular cartilage lesions: 5- to 11-year follow-up. Am J Sports Med 2014;42:912-20. [Crossref] [PubMed]
- Farr J 2nd. Autologous chondrocyte implantation and anteromedialization in the treatment of patellofemoral chondrosis. Orthop Clin North Am 2008;39:329-35. vi. [Crossref] [PubMed]
- Bellemans J, Cauwenberghs F, Brys P, et al. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med 1998;26:300-2. [Crossref] [PubMed]
- Stetson WB, Friedman MJ, Fulkerson JP, et al. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med 1997;25:570-4. [Crossref] [PubMed]
- Eager MR, Bader DA, Kelly JD 4th, et al. Delayed fracture of the tibia following anteromedialization osteotomy of the tibial tubercle: a report of 5 cases. Am J Sports Med 2004;32:1041-8. [Crossref] [PubMed]
- Torga Spak R, Teitge RA. Fresh osteochondral allografts for patellofemoral arthritis: long-term followup. Clin Orthop Relat Res 2006;193-200. [Crossref] [PubMed]
- Gomoll AH, Farr J, Hinckel B. Patellofemoral Osteochondral Allograft Transplantation. Oper Tech Sports Med 2015;23:150-6. [Crossref]
- Shubin Stein BE, Brady JM, Grawe B, et al. Return to Activities After Patellofemoral Arthroplasty. Am J Orthop (Belle Mead NJ) 2017;46:E353-7. [PubMed]
Cite this article as: Post WR. “Conservative” surgical treatment for anterior knee pain. Ann Joint 2018;3:68.


