
Efficacy of corticosteroid injection for subacromial impingement syndrome
Introduction
The ability of subacromial corticosteroid injections to alleviate pain and increase function in patients with shoulder pain is not clearly demonstrated by the orthopedics literature. Shoulder pain is extremely common and can be functionally debilitating, affecting patients of all ages and activity levels. Subacromial impingement syndrome (SIS) is the most common cause for shoulder pain (1,2). SIS is defined as compression and mechanic abrasion of rotator cuff tendons, long head of biceps tendon and subacromial bursa under the coracoacromial arc during arm elevation (3). Subacromial corticosteroid injections may be used as a palliative treatment option when other conservative treatments such as rest, non-steroidal anti-inflammatory drugs (NSAIDs), physical therapy, cryotherapy, ultrasound, electromagnetic radiation and activity modification have failed (4-6). Despite numerous randomized control trials and systematic reviews, the effectiveness of corticosteroid injections remains unknown. In a recent meta-analysis of randomized control trials, subacromial corticosteroid injection was reported to not reduce pain intensity any more than a placebo (7). Nevertheless many authors demonstrated good short term pain relief when used alongside other conservative treatment measures such as NSAIDs and therapeutic exercise (8,9). Due to the lack of supportive evidence in the current literature, the American Academy of Orthopedic Surgeons (AAOS) clinical practice guidelines for optimizing the management of rotator cuff conditions cannot recommend for or against the use of subacromial corticosteroid injections (10).
Despite the lack of supportive evidence, subacromial corticosteroid injections remain a standard practice in orthopedic care. The purpose of this study is to address the inconsistency in the literature by investigating whether corticosteroids provide significant short-term pain relief in the clinical setting utilizing pain and patient reported outcome measures (PROMs). We hypothesized that subacromial corticosteroid injections will relieve subacromial impingement syndrome related pain for a period of three months’ post-injection, but that the benefit will not carry over to six months’ post-injection. Our findings have the potential to provide evidence to support the way subacromial injections are currently used in the clinical setting to treat SIS.
Methods
The study was approved by our Institutional Review Board (IRB #2013P001198) and study participants were prospectively enrolled from the senior author’s clinic. All subjects who presented to the clinic with subacromial impingement syndrome and were both offered and decided to undergo a subacromial corticosteroid injection for temporary shoulder pain relief were asked to participate in the study. Inclusion/exclusion criteria were described, and if the patient was eligible and interested in participating, the study consent followed by the pre-injection survey were completed prior to the injection.
The inclusion criteria for the study were: (I) diagnosis of subacromial impingement syndrome (II) positive Neer and Hawkins signs (III) greater than 35 years old (IV) radiograph of affected shoulder within the past year and (V) English speaking. The exclusion criteria were: (I) prior subacromial injection of a corticosteroids within previous 1 year of the affected shoulder (II) prior shoulder surgery of the affected shoulder (III) current use of prescription narcotic pain medication (IV) systemic disease (i.e., rheumatoid arthritis or Lupus) (V) metabolic disease (i.e., Paget’s disease) (VI) pain disorder (i.e., Fibromyalgia, Reflex Sympathetic Dystrophy) (VII) cervical radiculopathy (VIII) arthritis (IX) fracture of the affected shoulder and (X) pregnancy.
Participants were recruited at the time of subacromial corticosteroid injection. They received one subacromial injection from a posterior portal while seated with 2 mL of Kenalog-10® (triamcinolone acetonide; Bristol Meyers Squibb) and 6 mL of 1% lidocaine (Hospira Inc.) without epinephrine. All injections were performed by a board-certified and fellowship trained orthopedic surgeon. All patients were encouraged to enroll in physical therapy following their corticosteroid injection. Pre-injection surveys were administered before the injection. Data was also collected at 2-week, 3- and 6-month after the date of injection. The PROMs collected were the Western Ontario Rotator Cuff index (WORC) (11). Shoulder Pain and Disability index (SPADI) (12), 36-Item Short Form Survey (SF-36), and Visual Analog Scale (VAS) for pain (13). At the 2-week time point, the only outcome collected was VAS pain score. Demographic data including age, sex, BMI, duration of symptoms, and smoking status were collected at the time of enrollment.
The pre-injection survey was completed in the office on an iPad and the 2-week, 3- and 6-month post-injection surveys were collected and managed using REDCap (Research Electronic Data Capture), which is a secure web-based application designed to support data capture for research studies (14).
Statistical analysis
The nonparametric Wilcoxon rank-sum test was used to determine whether differences in PROMs were statistically significant between pre-injection and 3-month and between pre-injection and 6-month. Statistical significance was obtained at an alpha level of 0.05. All statistical analysis was conducted in Stata Statistical Software (StataCorp., College Station, TX., 2015).
In addition to statistical significance, the minimally clinically important difference (MCID) was assessed for all PROMs that had established MCID values in the literature. The VAS pain scale and SPADI had available MCID values in the literature. For the VAS, an MCID value of 1.7 points was used for assessing the clinical impact of the results (13). For SPADI, a change of 8 points was used as the MCID value (15). There was no MCID established for WORC in the literature, although the minimally important difference (MID) has been defined as an 11.7% change in total score (11). No established MCID or MID was available for the SF-36 questionnaire based on our literature search.
Results
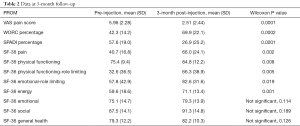
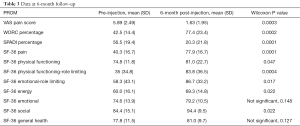
There were 34 patients enrolled in the study with a mean age of 51.3 years and an average of over 5 months of shoulder symptoms. Twenty-three patients completed the 3-month survey, giving a 68% follow-up rate at 3 months. Twenty patients completed the 6-month survey, giving a 59% follow-up rate at 6 months. There was a significant difference in sex/gender of the cohort which was 14.7% male (n=5) and 85.3% female (n=29). Twenty-nine (85.3%) patients enrolled in physical therapy during the study and 5 (14.7%) patients never enrolled in physical therapy. The demographic characteristics for the whole cohort, the cohort of patients who completed the 3-month follow-up, and the cohort of patients who completed the 6-month follow-up are listed in Table 1 respectively. Complete pre-injection versus 3-month follow-up PROM data can be seen in Table 2, and complete pre-injection versus 6-month follow-up PROM data can be seen in Table 3. The VAS pain score (0 being no pain, 10 being the worst pain possible), WORC percentage, SPADI percentage, and the SF-36 subsections of pain, physical functioning, physical function-role limiting, emotion-role limiting, and energy were all statistically significantly improved at both 3- and 6-month. Additionally, the VAS pain score was significantly reduced at the 2-week post-injection time point compared to the pre-injection time point from a mean VAS pain score of 5.9 at pre-injection to 2.5 at 2-week post-injection, as seen in Table 4.

Full table
Full table
Full table

Full table
The average change in VAS pain score at each study timepoint meet the MCID value of at least 1.73 cm change on a 10 cm scale (13). At 2-week, 66% of patients reported a change in VAS pain that exceeds the MCID change of 1.7/10. The percentage of patients who reported a change in VAS pain from pre-injection that exceeds the MCID were 70% and 78% at 3- and 6-month respectively. While there is not a MCID established for WORC, our results reach the minimally important difference (MID) of an 11.7% change in total score at each study time point (11). Finally, the MCID for SPADI has been established as a change of 8 points and our results reach this MCID at each study timepoint (15) (Tables 2,3).
Discussion
Providing evidence for standard clinical practice is an obligation for all physicians. The current studies assessing the efficacy of subacromial corticosteroid injections to temporarily relieve shoulder pain are insufficient. Due to the combination of a lack of consensus and paucity of high level studies investigating subacromial corticosteroid injections, the AAOS clinical practice guidelines deemed corticosteroid injection efficacy as “inconclusive”. The literature demonstrates conflicting results and lack strong evidence and objectivity in duration when assessing patient outcomes after subacromial corticosteroid injections (8,9,16,17). Akgün et al. found that injections were beneficial for short term pain relief (3-month) when used alongside NSAIDs and therapeutic exercise in an acute phase of SIS. However, this study lacked a significant sample size and failed to utilize a global health quality measure such as the SF-36. Blair et al. concluded short term relief was achieved but had a wide range of follow-up duration and failed to collect outcomes from patients at well-defined time points. A more objective study with consistent follow up durations is necessary. Vecchio et al. concluded that subacromial corticosteroid injections and controls had no statistically significant difference in regards to pain relief (18). A meta-analysis of randomized control trials of corticosteroid injection for rotator cuff tendinosis compared with placebo injection demonstrated that a corticosteroid injection did not reduce pain intensity more than a placebo injection at the 3-month assessment (7). The review of randomized control trials by Buchbinder et al. suggested that subacromial corticosteroid injection provided a small benefit over placebo in some trials however found no benefit of corticosteroid injection over NSAID use in a pool of three trials (6). In contrast, a systematic review by Arroll et al. reported significant improvement of symptoms with subacromial corticosteroid injections versus placebo for shoulder pain (19).
This study addresses some of our concerns with the current literature by utilizing validated PROMs at distinct post-injection time points. At 3-month, there was a statistically significant improvement of pre-injection WORC percentage, SPADI percentage, and the SF-36 subsections of pain, physical functioning, physical function-role limiting, emotional-role limiting and energy. At 6-month, there was a statistically significant improvement of pre-injection WORC percentage, SPADI percentage, and the SF-36 subsections of pain, physical functioning, physical function-role limiting, emotional-role limiting, energy and social. Contrary to our original hypothesis that the benefit of a corticosteroid injection would not carry over to the 6-month mark, patients still reported significant improvement on average at 6-month post-injection. The significant improvement in pain and function at both 3- and 6-month supports the practice of treating SIS with subacromial corticosteroid injections. This is in keeping with the results of the systematic review by Arroll et al. who also demonstrated a significant improvement in symptoms after treatment with a subacromial corticosteroid injection. It is also consistent with the results of Akgün et al. and Blair et al., both of which reported alleviating pain in the short term. For the VAS pain scale and the average change in VAS pain score exceeds the MCID at 2-week, 3-, and 6-month post-injection. For the SPADI, the average change exceeds the MCID at both 3- and 6-month and for the WORC, the average change exceeds the MID at 3- and 6-month post-injection.
The major limitations to this study are the small sample size and lack of control group. Future studies should aim to have a larger sample size to allow for stronger conclusions. Another potential limitation of this study is patient participation in physical therapy. While it is difficult to determine if the improvement in symptoms were due to injection combined with physical therapy or physical therapy alone, the injection was critical to patients previously in too much pain to participate in a physical therapy program. The significant initial drop in VAS scores at 2-week post-injection from 5.9/10 to 2.9/10 was likely due to the corticosteroid injection. This improvement in pain allowed patients to participate fully in a physical therapy program and ultimately improve their function, pain and range of motion in the affected shoulder.
Conclusions
The results of this study support the practice of treating SIS with subacromial corticosteroid injections in the clinical setting given the significant improvement in pain and function at both 3- and 6-month, as measured by the VAS pain score, WORC, SPADI, and SF-36 subsections of pain, physical functioning, physical functioning role limiting, emotional role limiting, and energy. The improvements in VAS pain and SPADI meet the MCIDs for these scores at all study time points and the WORC meets the MID at all study time points lends support to the clinical significance of these findings.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.07.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by our Institutional Review Board (IRB #2013P001198) and study participants were prospectively enrolled from the senior author’s clinic and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neer CS 2nd. Impingement Lesions. Clin Orthop Relat Res 1983;70-7. [PubMed]
- Fongemie AE, Buss DD, Rolnick SJ. Management of shoulder impingement syndrome and rotator cuff tears. Am Fam Physician 1998;57:667-74, 680-2. [PubMed]
- Göksu H, Tuncay F, Borman P. The comparative efficacy of kinesio taping and local injection therapy in patients with subacromial impingement syndrome. Acta Orthop Traumatol Turc 2016;50:483-8. [Crossref] [PubMed]
- Downing DS, Weinstein A. Ultrasound therapy of subacromial bursitis. A double blind trial. Phys Ther 1986;66:194-9. [Crossref] [PubMed]
- Rocks JA. Intrinsic shoulder pain syndrome: rationale for heating and cooling in treatment. Phys Ther 1979;59:153-9. [Crossref] [PubMed]
- Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev 2003;CD004016 [PubMed]
- Mohamadi A, Chan JJ, Claessen FMAP, et al. Corticosteroid Injections Give Small and Transient Pain Relief in Rotator Cuff Tendinosis: A Meta-analysis Clinical Orthopaedics and Related Research ®. Clin Orthop Relat Res 1999;232-43.
- Akgün K, Birtane M, Akarırmak Ü. Is local subacromial corticosteroid injection beneficial in subacromial impingement syndrome? Clin Rheumatol 2004;23:496-500. [Crossref] [PubMed]
- Blair B, Rokito AS, Cuomo F, et al. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am 1996;78:1685-9. [Crossref] [PubMed]
- Pedowitz RA, Yamaguchi K, Ahmad CS, et al. Optimizing the management of rotator cuff problems. J Am Acad Orthop Surg 2011;19:368-79. [Crossref] [PubMed]
- Kirkley A, Griffin S, Dainty K. Scoring Systems for the Functional Assessment of the Shoulder. Arthroscopy 2003;19:1109-20. [Crossref] [PubMed]
- Breckenridge JD, McAuley JH. Shoulder Pain and Disability Index (SPADI). J Physiother 2011;57:197. [Crossref] [PubMed]
- Tashjian RZ, Deloach J, Porucznik CA, et al. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg 2009;18:927-32. [Crossref] [PubMed]
- Obeid JS, McGraw CA, Minor BL, et al. Procurement of shared data instruments for Research Electronic Data Capture (REDCap). J Biomed Inform 2013;46:259-65. [Crossref] [PubMed]
- Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: A systematic review of four questionnaires. Arthritis Rheum 2009;61:623-32. [Crossref] [PubMed]
- Alvarez CM, Litchfield R, Jackowski D, et al. A Prospective, Double-Blind, Randomized Clinical Trial Comparing Subacromial Injection of Betamethasone and Xylocaine to Xylocaine alone in Chronic Rotator Cuff Tendinosis. Am J Sports Med 2005;33:255-62. [Crossref] [PubMed]
- McInerney JJ, Dias J, Durham S, et al. Randomised controlled trial of single, subacromial injection of methylprednisolone in patients with persistent, post-traumatic impingment of the shoulder. Emerg Med J 2003;20:218-21. [Crossref] [PubMed]
- Vecchio PC, Hazleman BL, King RH. A double-blind trial comparing subacromial methylprednisolone and lignocaine in acute rotator cuff tendinitis. Br J Rheumatol 1993;32:743-5. [Crossref] [PubMed]
- Arroll B, Goodyear-Smith F. Corticosteroid injections for painful shoulder: a meta-analysis. Br J Gen Pract 2005;55:224-8. [PubMed]
Cite this article as: Garvey KD, Solberg MJ, Cai A, Matzkin EG. Efficacy of corticosteroid injection for subacromial impingement syndrome. Ann Joint 2018;3:62.


