Periprosthetic humerus fractures: classification, management, and review of the literature
Introduction
Total shoulder arthroplasty (TSA) has been accepted as a reliable and predictable treatment option in the management of end-stage glenohumeral joint disease, with the frequency of the procedure increasing 2.5 folds over the past decade (1-3). Current evidence shows that primary, anatomic TSA improves both pain and function in up to 90–95% of patients (4-6). More recently, reverse total shoulder arthroplasty (rTSA) has also been shown to be effective in treating rotator cuff arthropathy, as well as failure of primary anatomic TSA (7-15). Long-term data demonstrate that these improvements in pain and function persist, and survivorship of these implants at ten years and twenty years is over 90% and 80%, respectively (10,16,17).
Despite this promising data, a wide-array of complications has been reported in the literature. Total complication rate for TSA/rTSA ranges from 3.6% to 20%, with one study examining rTSA noting a 68% complication rate (6,18-22). These complications include, but are not limited to, infection, instability, periprosthetic humerus fractures, neurovascular injury, component loosening, rotator cuff tear, and deltoid injury (20,23,24). Anatomic TSA complications include glenoid component loosening, osteolysis around either the humeral or glenoid component, instability from subscapularis failure all of which can result in significant bone lose. Additional complications seen more often in the setting of rTSA include baseplate failure, scapular/acromial stress fractures, instability, and component dissociation (14,15,25). Scapular notching is often frequently reported as a complication following rTSA, but the clinical significance of this is still unknown as there has be no study to demonstrate correlation with severity of notching and patient reported outcomes. While earlier data suggested a higher overall complication rate in rTSA when compared to anatomic TSA, more recent evidence suggests this may not be the case (13,26). The majority of investigations reporting complication rates following rTSA included a heterogenous cohort of patients with a variety of preoperative shoulder pathology including massive rotator cuff tears/rotator cuff tear arthropathy, primary osteoarthritis, post traumatic arthritis, revision shoulder arthroplasty, inflammatory arthritis, and fracture (27). Additionally, Jacxsens et al. (28) demonstrated that the significant amount of heterogeneity with regards to classifying and reporting complications makes comparison difficult, and a standardized scheme would be beneficial. The incidence of complications requiring reoperation is 5–11% and has been shown to be more frequent following revision procedures (18,29-31). A recent review article by Bohsali et al. reports an overall downward trend in complications over the last decade overall (27).
Periprosthetic humerus fractures are of particular importance, as they frequently lead to the need for revision surgery (32,33). These fractures can take place intraoperatively or postoperatively, occurring 0.9–3.5% and 1.0–3.0% of the time, respectively (20,21,25,27,29,32,34-38). They are more frequent during revision procedures, especially during extraction of a well fixed humeral stem (27,39-42). Preparing the humerus for an implant renders the bone more susceptible to fracture, and evidence shows that periprosthetic humerus fractures have a higher nonunion rate that native humerus fractures (6,43,44). This review, focuses on classification systems, risk factors, non-operative as well as operative management strategies, complications, and outcomes for both intraoperative and postoperative periprosthetic humerus fractures in the setting of anatomic and rTSA.
Classification
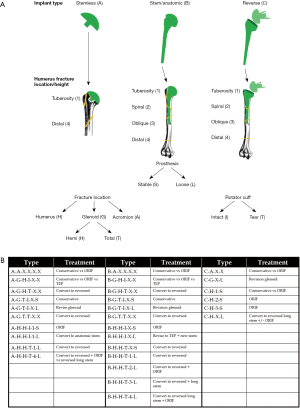
Wright and Cofield were the first to devise a classification system for postoperative periprosthetic humerus fractures in 1995 (43). They reported on a series of nine patients, and each was classified based on fracture pattern in relation to the distal tip of the prosthesis (Figure 1). While both types A and B fractures were centered at the tip, they differed in the amount of proximal extension. Type C fractures involved the humeral shaft distal to the tip of the implant. Type A fractures required surgery, with the fixation strategy dependent on implant stability. Long oblique or spiral type B fractures could be managed without surgery, while transverse or short oblique fractures fared better with operative intervention. Finally, type C fractures could be managed similarly to shaft fractures in the native humerus. This scheme has been shown to have good intraobserver (mean kappa, 0.69; range, 0.52–0.89) but poor interobserver reliability (mean kappa, 0.37; range, 0.24–0.50) (45). In 1999, Worland et al. published a similar classification scheme based on six patients, but subdivided type B fractures into B1-B3 based on fracture pattern and implant stability (46). B1 fractures are spiral fractures with a stable prosthesis, B2 are short oblique or transverse fractures around the tip of the stem and are also stable, and B3 are fractures resulting in an unstable stem. They noted that types A and C behave similar to native humerus fractures, while type B fractures present more of a treatment challenge depending on the stability of the stem. Groh et al. presented a similar classification system in 2008 developed from 15 patients which was also entirely based on the location of the fracture (47). Type I fractures occurred at the tip of the prosthesis, type II the fracture extended from proximal humeral shaft to beyond the distal aspect of the stem, and type III were fractures distal to the tip of the prosthesis.
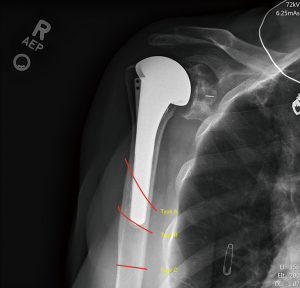
Campbell et al. classified fractures as belonging to one of four regions (Figure 2) (36). They assumed that fractures in the proximal humeral metaphysis have different implication for both healing and prosthetic stability than those occurring in the diaphysis. Of the 21 fractures in their series, 16 occurred intraoperatively. They concluded that intraoperative fractures all required anatomic reduction and stable intramedullary fixation. Region 1 and 2 fractures can be treated with standard length prosthetic stem and supplemental suture or cerclage wire fixation. Region 2 and 4 fractures required conversion to a long-stem implant with supplemental cerclage wiring.
Duncan et al. presented a classification system that could be broadly applied to any periprosthetic fracture which the entitled the “Unified Classification System” (48). This classification is based on whether the implant is well fixed, the patient’s bone quality, location with relation to soft tissue attachments, and whether the bone supports two joint replacements (i.e., a periprosthetic fracture between a total elbow and TSA). Its clinical utility in the classification and management of periprosthetic humerus fractures has not been well-elucidated in the literature as of this time.
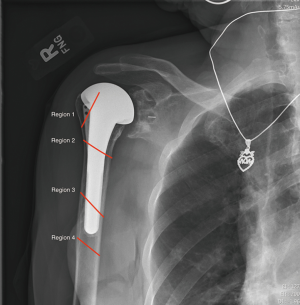
The most comprehensive classification system for periprosthetic humerus fractures to date was published in 2016 and validated in 2018 (49,50). Kirchhoff et al. aimed to create a structured approach to classifying periprosthetic humerus fractures that could be used to guide treatment in an algorithmic fashion (Figure 3A,B). Unlike previous iterations, this system takes into account the type of humeral prosthesis (stemless vs. anatomic vs. reversed), status of the rotator cuff, location of fracture, fracture pattern, and implant stability.
Risk factors
Patient factors
There have been several identified risk factors for both intraoperative and postoperative periprosthetic humerus fractures during TSA/rTSA. Intraoperative fractures most commonly occur during stem extraction during revision and most often involve the greater tuberosity, as this bone is often thin from stress shielding in the presence of a prior implant. Glenoid exposure in a shoulder that is severely retroverted or severe soft tissue contracture places the humeral shaft at increased risk for spiral diaphyseal fracture. Finally, overstuffing the humeral canal with a press fit humeral stem can result in metaphyseal fractures of the proximal humerus during impaction of the implant. Singh et al. performed a retrospective case series identifying female sex and posttraumatic glenohumeral arthritis as two risk factors for intraoperative periprosthetic humerus fractures (40). The authors hypothesized that the higher prevalence of osteoporosis in elderly females could explain this finding. Prior hemiarthroplasty and a history of instability have also been shown to increase risk of intraoperative humerus fractures (51). Higher number of medical comorbidities, increasing age, female sex, osteopenia, avascular necrosis, and rheumatoid arthritis are other risk factors that have been associated with an increased likelihood of postoperative humerus fractures (6,25,32,34,36,43,52-56).
Intraoperative factors
In addition to patient related factors, which often cannot be modified, there are multiple technical considerations a surgeon must be aware of in order to decrease the risks of a periprosthetic humerus fracture. Initially, it was believed that cementless implants conferred a high probability of fracturing the humerus, similar to press-fit femoral implants, but most recent evidence reveals no difference between cemented and cementless prostheses (37,40,57,58). Avoiding: (I) excessive external rotation of the humerus during reaming and/or broaching; (II) overzealous reaming (ream by hand); (III) under-reaming; (IV) malalignment of the stem; and (V) inserting oversized broach/prostheses are important technical aspects which have been shown to increase the risk for fracture (6,29,36,54,58). Appropriate patient positioning, ensuring adequate exposure with adequate soft tissue and capsular releases are imperative in avoiding these fractures, with some evidence hinting that an anterosuperior lateral approach is safer than the more traditional deltopectoral approach when performing rTSA—although this remains controversial (6,34,59). In revision procedures, periprosthetic humerus fracture frequently occurs either during implant removal or insertion; the remainder being during reaming or broaching (51,60). Most commonly the greater tuberosity is fractured during stem extraction or exposure of the glenoid.
Clinical evaluation and management
Intraoperative fractures
We will begin by reviewing the management strategies for intraoperative periprosthetic humerus fractures. We elected to discuss this separately as, assuming the fracture is identified during surgery, its treatment algorithm often differs significantly when compared to fractures discovered during follow up. Once a periprosthetic humerus fracture has occurred during surgery, several factors must be considered to determine the best strategy on how to proceed. These factors include location of fracture, fracture displacement, and bone quality. The goal is to employ techniques of rigid internal fixation, to prevent the fracture affecting the patient’s postoperative rehabilitation (58). Postoperative changes in rehabilitation are most significantly influence by fracture location, those involving the greater tuberosity and rotator cuff insertion need to proceed cautiously to allow for healing without displacement by the pull of the tendons. Fractures more distal involving the metaphysis or diaphysis may allow for immediate mobilization as would occur in a primary setting as often times a stable, rigid construct with long humeral stems supplemented with cables or suture can be achieved.
For nondisplaced fractures of the greater tuberosity that do not extend distally, observation alone is often adequate (6,37). If there is any displacement, internal fixation with suture or cerclage wiring is recommended (25,47). Normal postoperative rehab protocol can then be utilized for these patients. Surgical fixation is required if the tuberosity fracture extends into the proximal aspect of the humeral shaft or if it involves the surgical neck. If the bone quality is deemed reasonable, treatment with either suture or wire fixation and revision to long stemmed component or standard length prosthesis (if it extends at least 3 cortical diameters past fracture site) combined with inter-fragmentary screws is adequate (36). Bone graft can be utilized in cases of bone loss (37).
Treatment of shaft fractures depends on its location relative to the tip of the prosthesis. For fractures proximal to the tip of the prosthesis, revision to long stem implant, which extends at least two cortical diameters past the fracture site, with cerclage wire or cable fixation with or without allograft struts is recommended (6,36,37,47,58). Fractures distal to the prosthesis, but the prosthesis is deemed unstable, the same management strategy applies. If the prosthesis is deemed stable intraoperatively, the implant can be retained and internal fixation with hybrid plate/cerclage wire construct can be utilized (47,58).
Postoperative fractures
Preoperative evaluation
A careful history and physical examination should be performed on every patient presenting with a periprosthetic humerus fracture. A majority of these are caused by low-energy mechanisms such as fall from standing and determining pre-injury functional status can help guide subsequent treatment. It is important to determine patients whether or not pain was present prior to the fracture, as that could be a sign of implant loosening or low virulence infection such as propionibacterium acnes. Although inflammatory markers such as ESR/CRP are valuable in evaluating for an occult infection, these studies are often unreliable in the setting of an acute fracture. Presence of bacteria can be better determined at time of revision surgery, but significance of unexpected positive cultures continues to be investigated. Medical records are helpful in determining preoperative range of motion, identifying surgical approach, as well as confirming which implants are present. Knowledge of what implant is being revised is helpful for preoperative planning as well as nuances of the system with regards to extraction. Physical examination is often pain-limited, but the condition of the skin, prior scars, as well as neurovascular status should be carefully evaluated and accurately documented. It is important to evaluate the axillary and radial nerves specifically as these neurologic structures are at particular risk for injury depending on the location of the periprosthetic fracture and zone of injury.
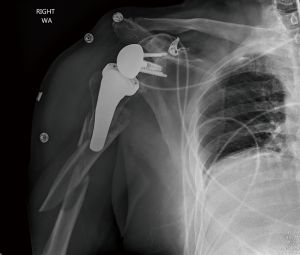
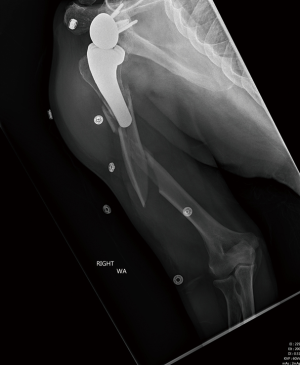
AP, true AP, scapular-Y, and axillary lateral views (Figure 4) of the shoulder should be obtained, in addition to full length AP and lateral humerus XRs (Figure 5). CT scan is indicated to better determine extent of the fracture as well as evaluating humeral and glenoid bone stock (56). It also provides valuable information regarding glenoid morphology and remaining glenoid vault. CT arthrogram or ultrasound can be helpful to assess the status of the rotator cuff and CT arthrogram can provide additional details on stability of the components especially the glenoid in the setting of anatomic TSA.
Nonoperative management
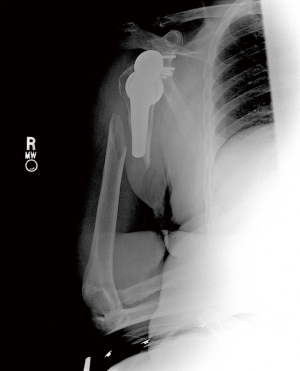
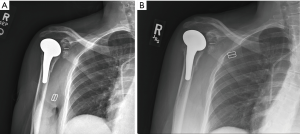
Nonoperative management has been shown to be successful when appropriately indicated (Figure 6A,B). Multiple studies show that fractures distal to a well-fixed prosthesis (type C) can be treated nonoperatively similar to native humeral shaft fractures (43,61-64). Additionally, if the fracture is proximal to the tip of a well-fixed implant, a trial of non-operative management is reasonable—although this remains controversial (33,43,61). Acceptable closed reduction parameters are similar to native humerus fractures (63). However, if the prosthesis is loose, surgical intervention is indicated—assuming the patient is medically fit for surgery.
Operative management
While heterogeneity remains in the literature, commonly agreed upon indications for surgical treatment of periprosthetic humerus fractures are displaced/unstable fractures and fractures around a loose humeral component (45,49,65).
The first step when planning treatment is to determine implant stability. Preoperative XRs can be evaluated using criteria presented by Sperling et al. and Sanchez-Sotela et al. (66,67). They determined that humeral implants with >2 mm of lucency in at least 3 of 8 humeral zones or those where 2 of 3 independent observers believed there to be subsidence or tilt of the implant were at increased risk for loosening (Figure 7). However, the most accurate method to determine implant stability is intraoperatively. This can be performed by applying a direct force to the humeral stem tip after exposure of fracture site (45,68). If equivocal, proximal humerus exposure can be performed by taking down subscapularis tendon and capsule, and the bone-implant interface can be directly observed while again applying pressure to the stem tip through the fracture site. Alternatively, the rotator interval can be opened proximally to facilitate the evaluation of the stem at the level of the glenohumeral joint (68).
Recent data show that type A fractures can be treated nonoperatively if they are nondisplaced (25,29). However, many believe these fractures should be managed with revision to long stem prosthesis with additional fixation determined by bone quality (plate, cerclage wiring) (63). Successful operative management of these fractures with just suture fixation without implant revision has been reported (20,29). Type B fractures with a stable implant can be treated with implant retention and osteosynthesis (25,33,65,68-70). Specific constructs vary widely in the literature, without significant difference in union rates. If the implant is loose intraoperatively, conversion to long stem prosthesis is treatment of choice. Implant should span 2 or 3 cortical diameters past the fracture site (33). Supplemental fixation with cerclage wire with or without allograft strut augmentation or plate constructs is often helpful (Figure 8). Augmentation with allograft is also an option (45). When there is significant proximal humeral bone loss from either the fracture, stem extraction or both proximal humerus composite allograft can be used. Type C fractures with a loose stem should be treated similarly. Type C fractures with a well-fixed stem can be treated similarly to native humeral shaft fractures. Of note, surgeons will likely have to utilize cables, wires, unicortical screws, if locked plating is used in these settings due to the presence of the prosthesis proximally or the humeral stem can be revised to a long stem construct (47,49,64,70-76) (Figure 9).
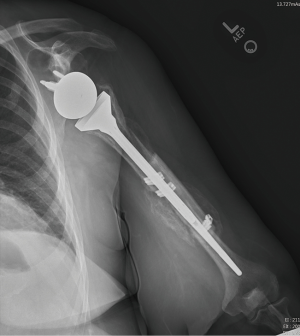
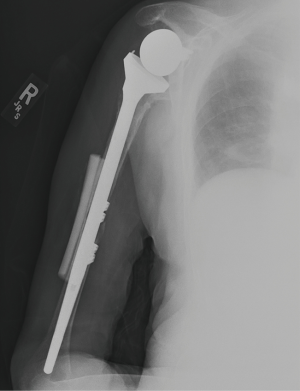
Outcomes
Overall union rate after surgery for periprosthetic humerus fractures is high, with one series reporting 97% union rate in 36 patients treated either with revision arthroplasty or ORIF (45). In the ORIF group (n=17), fractures healed at an average of 6.8 months. Of the 6 that had pre-fracture ASES scores, 5 returned to that level postoperatively. In the revision arthroplasty group (n=19), average time to union was 7.7 months. There was a statistically significant increase in preoperative versus postoperative ASES scores. Despite this promising date, they noted a 39% complication rate and 19% reoperation rate. Other studies similarly show high complication rates and note that patients do not do nearly as well as they did after their index procedure (43,53,56).
Summary/authors recommendation
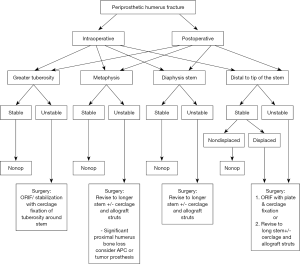
Timing, location, and implant stability of periprosthetic humeral fractures related to shoulder arthroplasty dictates treatment recommendations. While the published outcomes are limited to retrospective case series for evidence-based treatment decisions, we have developed a simple treatment algorithm to allow for best predictable functional outcomes (Figure 10).
Intraoperative humeral fractures occurring either primary or revision shoulder arthroplasty most commonly involve the greater tuberosity or humeral metaphysis. (I) Fractures of the greater tuberosity are addressed with high strength non-absorbable doubled wracking hitch suture technique where the suture is passed at the tendon bone junction of the supraspinatus/infraspinatus and secured around the humeral stem. (II) Metaphyseal humeral fractures typically occur during stem insertion or humeral preparation and can be treated with cerclage technique around the metaphysis.
Postoperative periprosthetic fractures can occur at any location along the humerus and as all of the classifications have outlined the most important factors include location and implant stability. We have simplified this into categories: can the stem be preserved, or does it need to be revised. A CT scan is always obtained and allows for perioperative assessment of remaining humeral bone as well as fracture pattern. (I) Proximal fractures with a stable implant are treated nonoperatively. (II) Fractures involving the metaphysis/diaphysis region with in the area of the stem are driven by remaining proximal humeral bone, stem stability, and method of fixation of primary stem (i.e., how destructive would it be to the remaining bone to extract and revise the prosthesis). (III) Stems that are grossly loose are revised to long stem humeral prosthesis with cerclage supplementation around the fracture with or without allograft struts. (IV) Stems that are stable are driven by fracture pattern, and how much of the stem is involved with the fracture.
Additional outcomes-based studies are needed to provide data on how these fractures effect implant survivorship and patient reported functional outcome measures.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: MK is a consultant and receives research support from Tornier/Wright Medical both of which are unrelated to the content of this work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim SH, Wise BL, Zhang Y, et al. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am 2011;93:2249-54. [Crossref] [PubMed]
- Roberson TA, Bentley JC, Griscom JT, et al. Outcomes of total shoulder arthroplasty in patients younger than 65 years: a systematic review. J Shoulder Elbow Surg 2017;26:1298-306. [Crossref] [PubMed]
- Baulot E, Sirveaux F, Boileau P. Grammont's idea: The story of Paul Grammont's functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res 2011;469:2425-31. [Crossref] [PubMed]
- Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg 2002;11:130-5. [Crossref] [PubMed]
- Buck FM, Jost B, Hodler J. Shoulder arthroplasty. Eur Radiol 2008;18:2937-48. [Crossref] [PubMed]
- Cameron B, Iannotti JP. Periprosthetic fractures of the humerus and scapula: management and prevention. Orthop Clin North Am 1999;30:305-18. [Crossref] [PubMed]
- Cho CH, Song KS, Koo TW. Clinical Outcomes and Complications during the Learning Curve for Reverse Total Shoulder Arthroplasty: An Analysis of the First 40 Cases. Clin Orthop Surg 2017;9:213-7. [Crossref] [PubMed]
- Boileau P, Gonzalez JF, Chuinard C, et al. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg 2009;18:600-6. [Crossref] [PubMed]
- Boileau P, Watkinson DJ, Hatzidakis AM, et al. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005;14:147S-61S. [Crossref] [PubMed]
- Cuff DJ, Pupello DR, Santoni BG, et al. Reverse Shoulder Arthroplasty for the Treatment of Rotator Cuff Deficiency: A Concise Follow-up, at a Minimum of 10 Years, of Previous Reports. J Bone Joint Surg Am 2017;99:1895-9. [Crossref] [PubMed]
- Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2009;17:284-95. [Crossref] [PubMed]
- Vanhove B, Beugnies A. Grammont's reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg 2004;70:219-25. [PubMed]
- Farshad M, Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop 2010;34:1075-82. [Crossref] [PubMed]
- Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect 2012;61:157-68. [PubMed]
- Russo R, Rotonda GD, Ciccarelli M, et al. Analysis of complications of reverse total shoulder arthroplasty. Joints 2015;3:62-6. [Crossref] [PubMed]
- Cil A, Veillette CJ, Sanchez-Sotelo J, et al. Survivorship of the humeral component in shoulder arthroplasty. J Shoulder Elbow Surg 2010;19:143-50. [Crossref] [PubMed]
- Favard L, Levigne C, Nerot C, et al. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res 2011;469:2469-75. [Crossref] [PubMed]
- Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:388-94. [Crossref] [PubMed]
- Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am 1996;78:603-16. [Crossref] [PubMed]
- Chin PY, Sperling JW, Cofield RH, et al. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg 2006;15:19-22. [Crossref] [PubMed]
- Zumstein MA, Pinedo M, Old J, et al. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146-57. [Crossref] [PubMed]
- Waterman BR, Dunn JC, Bader J, et al. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg 2015;24:24-30. [Crossref] [PubMed]
- Mayne IP, Bell SN, Wright W, et al. Acromial and scapular spine fractures after reverse total shoulder arthroplasty. Shoulder Elbow 2016;8:90-100. [Crossref] [PubMed]
- Caniggia M, Fornara P, Franci M, et al. Shoulder arthroplasty. Indications, contraindications and complications. Panminerva Med 1999;41:341-9. [PubMed]
- Garcia-Fernandez C, Lopiz-Morales Y, Rodriguez A, et al. Periprosthetic humeral fractures associated with reverse total shoulder arthroplasty: incidence and management. Int Orthop 2015;39:1965-9. [Crossref] [PubMed]
- Kiet TK, Feeley BT, Naimark M, et al. Outcomes after shoulder replacement: comparison between reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:179-85. [Crossref] [PubMed]
- Bohsali KI, Bois AJ, Wirth MA. Complications of Shoulder Arthroplasty. J Bone Joint Surg Am 2017;99:256-69. [Crossref] [PubMed]
- Jacxsens M, Walz T, Durchholz H, et al. Towards standardised definitions of shoulder arthroplasty complications: a systematic review of terms and definitions. Arch Orthop Trauma Surg 2017;137:347-55. [Crossref] [PubMed]
- Gonzalez JF, Alami GB, Baque F, et al. Complications of unconstrained shoulder prostheses. J Shoulder Elbow Surg 2011;20:666-82. [Crossref] [PubMed]
- Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am 2006;88:2279-92. [PubMed]
- Boileau P, Watkinson D, Hatzidakis AM, et al. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 2006;15:527-40. [Crossref] [PubMed]
- Ingoe HM, Holland P, Cowling P, et al. Intraoperative complications during revision shoulder arthroplasty: a study using the National Joint Registry dataset. Shoulder Elbow 2017;9:92-9. [Crossref] [PubMed]
- Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res 2016;102:S33-43. [Crossref] [PubMed]
- Cowling PD, Holland P, Kottam L, et al. Risk factors associated with intraoperative complications in primary shoulder arthroplasty. Acta Orthop 2017;88:587-91. [Crossref] [PubMed]
- Della Rocca GJ, Leung KS, Pape HC. Periprosthetic fractures: epidemiology and future projections. J Orthop Trauma 2011;25:S66-70. [Crossref] [PubMed]
- Campbell JT, Moore RS, Iannotti JP, et al. Periprosthetic humeral fractures: mechanisms of fracture and treatment options. J Shoulder Elbow Surg 1998;7:406-13. [Crossref] [PubMed]
- Athwal GS, Sperling JW, Rispoli DM, et al. Periprosthetic humeral fractures during shoulder arthroplasty. J Bone Joint Surg Am 2009;91:594-603. [Crossref] [PubMed]
- Capone A, Congia S, Civinini R, et al. Periprosthetic fractures: epidemiology and current treatment. Clin Cases Miner Bone Metab 2017;14:189-96. [Crossref] [PubMed]
- Steinmann SP, Cheung EV. Treatment of periprosthetic humerus fractures associated with shoulder arthroplasty. J Am Acad Orthop Surg 2008;16:199-207. [Crossref] [PubMed]
- Singh JA, Sperling J, Schleck C, et al. Periprosthetic fractures associated with primary total shoulder arthroplasty and primary humeral head replacement: a thirty-three-year study. J Bone Joint Surg Am 2012;94:1777-85. [Crossref] [PubMed]
- Matsen FA 3rd, Boileau P, Walch G, et al. The reverse total shoulder arthroplasty. J Bone Joint Surg Am 2007;89:660-7. [Crossref] [PubMed]
- Werner CM, Steinmann PA, Gilbart M, et al. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am 2005;87:1476-86. [PubMed]
- Wright TW, Cofield RH. Humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am 1995;77:1340-6. [Crossref] [PubMed]
- Choo AM, Hawkins RH, Kwon BK, et al. The effect of shoulder arthroplasty on humeral strength: an in vitro biomechanical investigation. Clin Biomech (Bristol, Avon) 2005;20:1064-71. [Crossref] [PubMed]
- Andersen JR, Williams CD, Cain R, et al. Surgically treated humeral shaft fractures following shoulder arthroplasty. J Bone Joint Surg Am 2013;95:9-18. [Crossref] [PubMed]
- Worland RL, Kim DY, Arredondo J. Periprosthetic humeral fractures: management and classification. J Shoulder Elbow Surg 1999;8:590-4. [Crossref] [PubMed]
- Groh GI, Heckman MM, Wirth MA, et al. Treatment of fractures adjacent to humeral prostheses. J Shoulder Elbow Surg 2008;17:85-9. [Crossref] [PubMed]
- Duncan CP, Haddad FS. The Unified Classification System (UCS): improving our understanding of periprosthetic fractures. Bone Joint J 2014;96-B:713-6. [Crossref] [PubMed]
- Kirchhoff C, Kirchhoff S, Biberthaler P. Classification of periprosthetic shoulder fractures. Unfallchirurg 2016;119:264-72. [Crossref] [PubMed]
- Kirchhoff C, Beirer M, Brunner U, et al. Validation of a new classification for periprosthetic shoulder fractures. Int Orthop 2018;42:1371-7. [Crossref] [PubMed]
- Wagner ER, Houdek MT, Elhassan BT, et al. What Are Risk Factors for Intraoperative Humerus Fractures During Revision Reverse Shoulder Arthroplasty and Do They Influence Outcomes? Clin Orthop Relat Res 2015;473:3228-34. [Crossref] [PubMed]
- Bonutti PM, Hawkins RJ. Fracture of the humeral shaft associated with total replacement arthroplasty of the shoulder. A case report. J Bone Joint Surg Am 1992;74:617-8. [Crossref] [PubMed]
- Boyd AD Jr, Thornhill TS, Barnes CL. Fractures adjacent to humeral prostheses. J Bone Joint Surg Am 1992;74:1498-504. [Crossref] [PubMed]
- McDonough EB, Crosby LA. Periprosthetic fractures of the humerus. Am J Orthop (Belle Mead NJ) 2005;34:586-91. [PubMed]
- Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med 2015;8:83-91. [Crossref] [PubMed]
- Sewell MD, Kang SN, Al-Hadithy N, et al. Management of peri-prosthetic fracture of the humerus with severe bone loss and loosening of the humeral component after total shoulder replacement. J Bone Joint Surg Br 2012;94:1382-9. [Crossref] [PubMed]
- Werthel JD, Lonjon G, Jo S, et al. Long-term outcomes of cemented versus cementless humeral components in arthroplasty of the shoulder: a propensity score-matched analysis. Bone Joint J 2017;99-B:666-73. [Crossref] [PubMed]
- Williams GR Jr, Iannotti JP. Management of periprosthetic fractures: the shoulder. J Arthroplasty 2002;17:14-6. [Crossref] [PubMed]
- Mole D, Favard L. Excentered scapulohumeral osteoarthritis. Rev Chir Orthop Reparatrice Appar Mot 2007;93:37-94. [PubMed]
- Wagner ER, Statz JM, Houdek MT, et al. Use of a shorter humeral stem in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1454-61. [Crossref] [PubMed]
- Terabayashi N, Matsumoto K, Takigami I, et al. Treatment of Humeral Fracture after Shoulder Arthroplasty using Functional Brace: A Case Report. J Orthop Case Rep 2016;6:3-5. [PubMed]
- Kim DH, Clavert P, Warner JJ. Displaced periprosthetic humeral fracture treated with functional bracing: a report of two cases. J Shoulder Elbow Surg 2005;14:221-3. [Crossref] [PubMed]
- Kumar S, Sperling JW, Haidukewych GH, et al. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am 2004;86-A:680-9. [Crossref] [PubMed]
- Mavrogenis AF, Angelini A, Guerra E, et al. Periprosthetic fractures of the humerus. J Long Term Eff Med Implants 2009;19:305-11. [Crossref] [PubMed]
- Jaeger M, Maier D, Izadpanah K, et al. Prosthesis replacement in periprosthetic humeral fractures. Oper Orthop Traumatol 2017;29:492-508. [Crossref] [PubMed]
- Sperling JW, Cofield RH, O'Driscoll SW, et al. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg 2000;9:507-13. [Crossref] [PubMed]
- Sanchez-Sotelo J, O'Driscoll SW, Torchia ME, et al. Radiographic assessment of cemented humeral components in shoulder arthroplasty. J Shoulder Elbow Surg 2001;10:526-31. [Crossref] [PubMed]
- Kurowicki J, Momoh E, Levy JC. Treatment of Periprosthetic Humerus Fractures With Open Reduction and Internal Fixation. J Orthop Trauma 2016;30:e369-e74. [Crossref] [PubMed]
- Christoforakis JJ, Sadiq S, Evans MJ. Use of a Dall-Miles plate and cables for the fixation of a periprosthetic humeral fracture. Acta Orthop Belg 2003;69:562-5. [PubMed]
- Kent ME, Sinopidis C, Brown DJ, et al. The locking compression plate in periprosthetic humeral fractures A review of two cases. Injury 2005;36:1241-5. [Crossref] [PubMed]
- Martinez AA, Calvo A, Cuenca J, et al. Internal fixation and strut allograft augmentation for periprosthetic humeral fractures. J Orthop Surg (Hong Kong) 2011;19:191-3. [Crossref] [PubMed]
- Mineo GV, Accetta R, Franceschini M, et al. Management of shoulder periprosthetic fractures: our institutional experience and review of the literature. Injury 2013;44:S82-5. [Crossref] [PubMed]
- Schoch B, Mehta S, Namdari S. Surgical Fixation of Periprosthetic Humerus Fractures Using an Extension Plate: Surgical Technique and Report of 5 Cases. J Orthop Trauma 2017;31:e432-5. [Crossref] [PubMed]
- Seybold D, Citak M, Konigshausen M, et al. Combining of small fragment screws and large fragment plates for open reduction and internal fixation of periprosthetic humeral fractures. Int J Shoulder Surg 2011;5:105-7. [Crossref] [PubMed]
- Wutzler S, Laurer HL, Huhnstock S, et al. Periprosthetic humeral fractures after shoulder arthroplasty: operative management and functional outcome. Arch Orthop Trauma Surg 2009;129:237-43. [Crossref] [PubMed]
- Thes A, Klouche S, de Tienda M, et al. Cortical onlay strut allograft with cerclage wiring of periprosthetic fractures of the humerus without stem loosening: technique and preliminary results. Eur J Orthop Surg Traumatol 2017;27:553-7. [Crossref] [PubMed]
Cite this article as: Gebrelul A, Green A, Schacherer T, Khazzam M. Periprosthetic humerus fractures: classification, management, and review of the literature. Ann Joint 2018;3:49.