
Anterior approach total hip replacement (THA) with a specialized orthopedic table
Background
The first hip arthroplasty performed through the anterior approach (AA) was done in 1947 by French surgeon Robert Judet with the assistance of the Judet-Tasserit table (a specialized orthopedic table) (1). Judet used the intermuscular interval between the sartorius and the tensor fascia lata (TFL), described as the Heuter interval. Although the posterior approach with osteotomy of the greater trochanter, as described by Charnley (2,3), initially overtook the AA regarding popularity and general acceptance amongst surgeons, the AA as seen a resurgence in recent years. A new orthopedic table, modern arthroplasty implants, fluoroscopy, computer navigation, improved postoperative recovery and most importantly patient demand have all in part played a role in its rise in popularity (4-8). The AA is performed by 34% of surgeons in the USA, according to a recent survey performed by the American Association of Hip and Knee Surgeons (9).
In the setting of hip arthroplasty, the AA was appealing to the senior author several benefits over other approaches including lower risk of dislocation (increased in the posterior approach) and greater preservation of tissues such as the abductor complex and the external rotators (anterolateral and posterior approaches, respectively) (4,10,11). These and other benefits are not limited to surgeons that chose to use the specialized orthopedic table, supine positioning, and fluoroscopic imaging. However, the senior author believes that with the use of these additional tools, the AA can be used to produce consistently excellent results for each total hip arthroplasty.
Technique
Positioning
Once the anesthesia has been induced (the senior author prefers spinal anesthesia without narcotics), the patient is placed supine on the Judet-type orthopedic table (Hana table, OSI, USA) specialized orthopedic table. The groin post is placed, and the arms are strapped to the arm boards at about 85°, then feet are placed into the padded boots and attached to the leg spars. Next, the spars are flexed 5° to accommodate for a pelvic extension that can occur when the patient is lying supine. The feet are rotated approximately 15° to position the proximal femur close to its position of greatest femoral offset and true anteversion (Figure 1). Finally, the pelvis is positioned firmly against the post, and gentle traction is placed on the legs, beginning with the non-operative extremity.
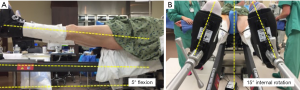
The patient is then prepped and draped. The extent of the draping is from the level of the umbilicus to mid femur and from the posterior aspect of the gluteus to the hip flexion crease. Care is taken to keep the femoral lift post from being covered by drapes except for the final, to allow later placement by cutting with a knife and then sealed with 3M™ Ioban™ 2 Incise Drape once the femoral elevator is in place.
Exposure
The AA is in the internervous plane of the superior gluteal nerve and the femoral nerve, and it is in the intermuscular interval of the TFL and sartorius superficially and the TFL and the rectus femoris (RF) deep. The incision is marked as starting ~2–4 cm lateral to the ASIS obliquely in line with the body of the TFL for a length of 9–10 cm (Figure 2). Dissection through the skin is performed sharply to the level of the fascia over the TFL, which typically has a violet-blue hue indicating that the initial dissection is not too anterior or too posterior (Figure 3). Care is taken to not undermine any tissue and to cauterize any bleeding vessels. This TFL fascia is incised sharply in line with the fibers of the TFL and extended beyond the length of the skin incision by 2–3 cm in both the proximal and distal direction. Next, an Allis clamp is used to hold the anterior limb of the fascia of the TFL while finger dissection is used to peel the medial fibers of the TFL off of this fascia. A key point to this finger dissection is to free the muscle off of the fascia proximally as well as distally to allow mobilization of the TFL facilitating the visualization and access to the joint later in the approach.
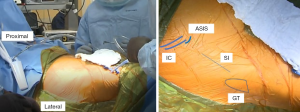
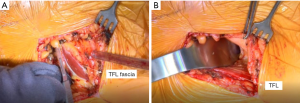
At this juncture, the surgeon can palpate the capsule over the anterior femoral neck. One can gently lift the femur while his or her finger is over the capsule to confirm that the finger is in the correct location. Next, a cobra retractor is placed over the superior neck, and a Hohmann retractor is placed inferiorly (Figure 3). It is important to avoid placing the retractors through the substance of the TFL or RF. A Meyerding or Hibbs is used to retract the TFL distal to the cobra retractor.
Now the lateral femoral circumflex vessels can be identified in the distal aspect of the incision. Typically, they run horizontally, almost perpendicular to the incision and are obscured by fat. The vessels can be better visualized by some gentle dissection with a tonsil clamp. Once they are visualized, they are cauterized (Figure 4). The senior author chooses to use the tonsil clamp to clamp them medially and then laterally and use the electrocautery device on the clamp. Care must be taken to cauterize completely, in particular, the artery, which is the distal-most vessel and can sometimes be missed. Next, there is a “fascia with no name” as described by Letournel overlying the distal capsule and the vastus lateralis (VL) that is cut using a Mayo scissor.
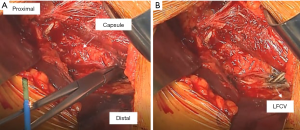
The capsule should be well visualized, and the landmarks for making the capsulotomy can be identified. The capsulotomy is performed with cautery beginning at the superior most corner that is visualized through the approach (Figure 5). It is then directed towards the lateral aspect of the anterior tubercle of the femur, which is located at the most proximal point of the IT (intertrochanteric) line. The capsulotomy then takes a sharp turn following the IT line. It is helpful to remove the capsule in its entirety off the bone, and it may require sacrificing a few fibers of the VL.
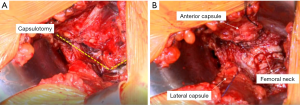
Next, the anterior limb is tagged with a 1-0 Vicryl suture; care is taken to exit the needle in the mid-substance of the capsule where it was cut. This is done to protect the suture from being caught in reamers or during calcar planning (Figure 5). Once tagged and clamped with a straight clamp, the Hohmann retractor is replaced with a cobra retractor underneath the capsule. Then, the posterior limb is tagged similarly, clamped with a curved clamp and finally, the cobra is moved from its extracapsular position to intracapsular.
Dislocation of hip joint
A critical part of achieving excellent visualization of the femur during the AA is to mobilize the femur fully and early. To do so, the femoral head is dislocated before osteotomy of the neck. This technique begins with a small, bent Hohmann is placed under the capsule to expose the lateral acetabulum and labrum. Then a 1 cm straight osteotome is used to remove any laterally overhanging osteophytes to facilitate placement of the hip skid (Figure 6). The preoperative radiograph can be referenced to see how much lateral bone to remove. The foot is rotated 45° outward, and the rotation is left unlocked.
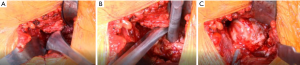
Traction is then placed on the leg (typically approximately three turns of the wheel) until there is some distraction of the hip joint to allow room for the hip skid. The hip skid used is shaped more like a large Cobb elevator with a T-shaped handle. This design allows getting the skid to “cup” the femoral head as well as to make cutting the ligamentum teres easier. Once the hip skid is placed, two turns of traction are taken off to make external rotation easier. A corkscrew is inserted centrally into the femoral head (it is important that its final position in the head is purely vertical to prevent impingement on the TFL when dislocated, both preventing injury to the TFL and allowing a more complete dislocation) (Figure 6). The head is dislocated in one smooth maneuver which involves vertical (towards the ceiling) traction on the corkscrew while simultaneously “scooping” and levering the femoral head out. When done properly the surgeon’s hands move in opposite directions of each other, and the head comes out with little effort. In the senior author’s experience, there have been no anterior wall fractures from lever the hip skid.
Once the femoral head is dislocated, the table operator externally rotated the leg as far as it can go. This allows exposure of the medial neck towards the less trochanter (LT) by placing a small, bent Hohmann under the VL both to aid in exposure but also to protect the neurovascular bundle supplying the VL muscle when the medial capsule is dissected off the bone towards the lesser trochanter (Figure 6). The cobra tractor inferior to the neck can be repositioned under the capsule after the initial, medial capsule is removed to allow better access to the remaining attached hip capsule towards the LT (Figure 7). Once the release is complete, the area is inspected for bleeding vessels. The bent Hohmann is removed, and the femoral head is then reduced with the corkscrew in place.
Osteotomy
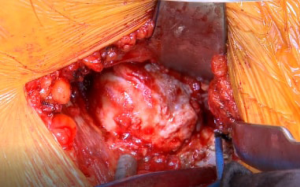
Preoperative templating is again evaluated intraoperatively to see the level of the neck cut (Figure 8). The critical landmark in making the neck cut is the “saddle” of the neck (the portion of the neck superiorly where the neck transitions into the GT) because that is the lateral level of the osteotomy. The senior author uses the Actis™ stem (DePuy Orthopaedics, Warsaw, IN, USA). This implant has a collar, and it requires a neck that is more horizontal than many other implants. An additional reason that the neck cut is initially more horizontal is that the preserved excess medial neck allows the surgeon to calcar plane the neck down to the exact level of where the surgeon desires the collar of the final implant to sit. This will be discussed in greater detail later when describing how accurate offset and lengths are achieved.
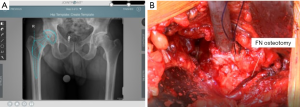
The two cobras and Meyerding retractors are placed in their previous configuration, as described above. An oscillating saw is used to cut the medial portion of the neck completely through from anterior to posterior. A straight, 1 cm osteotome is used to complete the superolateral portion of the osteotomy (Figure 8). An osteotome provides more control in the vulnerable area of the GT than an oscillating saw. The surgeon can see that the neck cut is complete because the bone distal to the cut falls away when the osteotomy is completed. The head is then removed using the retained corkscrew in a rolling motion carefully over the TFL to prevent any injury.
Acetabular exposure and reaming
The femur is externally rotated to 45° and rotation is locked. This position of the femur moves the neck out of the way for better visualization of the acetabulum. In the case of a neck cut that is too long, sometimes visualization can be improved with more than 45° of external rotation without having to make an additional cut. A small bent Hohmann retractor is placed over the anterior acetabulum. Care must be taken to place the tip of Hohmann over anterior acetabulum on bone and not to place the retractor too medial where there is a risk of injuring the femoral nerve. A cobra retractor is placed under the posterior aspect of the acetabulum at the 10 o’clock position for a right hip or two o’clock position for a left hip. The acetabulum is inspected. If visualization the posterior capsule is obstructed by the remaining femoral neck then the neck cut is too long and can be recut to improve exposure or rotated as described above. An episiotomy is performed in the posteroinferior capsule to decrease the tension of the posterior capsule. Any overhanging labrum is sharply excised.
An initial reamer (typically 44 mm) is placed and reamed to the medial aspect of the cotyloid fossa. The next reamer is 4–6 mm less then templated acetabular size. It is directed medial and approximately 15° in the anterior to posterior direction (Figure 9). The retractors are removed, and a 9-inch fluoroscopy machine is then used to get a posteroanterior (PA) radiograph of the pelvis. The specialized table is repositioned (to correct for pelvic roll) under direction from the surgeon to obtain a true PA pelvis image. Care is taken to recreate pelvic position as it appears on the preoperative supine AP radiograph. Once the initial PA has been obtained, reaming is begun under fluoroscopic guidance. Reaming is performed up to the templated size. Periodically during reaming, the anterior and posterior walls of the acetabulum are digitally palpated to confirm that there is adequate coverage of the acetabular component. Of note, the final reamer is directed in a more superior direction, and the reaming technique consists of pulsing the reamer until the torque gives way to a full rotation of the grater. The final reamer is the same size as the acetabular component, as the senior surgeon places the cup using a “line to line” technique with no screw fixation.
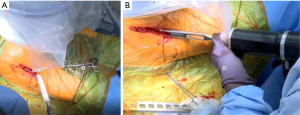
The senior author uses computer navigation with JointPoint™ software (Jointpoint, Inc., Belleair Bluffs, FL, USA) for both the acetabulum and the femur. On the acetabular side, the benefit of the software, in addition to what is offered by the traditional fluoroscopy views, is that it draws an ellipse at the inclination and version of the cup. This is desired by the surgeon on the screen that can be used as a target as well as to assess the anteversion and inclination of an intact cup. However, it is important to note that the acetabulum should be centered on the screen to prevent the effects of parallax in misrepresenting the cup angle in addition to having a PA imaging of pelvis or hip in a neutral rotation to the plane of the fluoroscopy device.
The final acetabular component (Pinnacle™ Triflange Acetabular System, DePuy Orthopaedics, Inc, Warsaw, IN, USA) is impacted using the ME1000™ Surgical Impactor (Medical Enterprises Distribution). This is a device that uses compressed air to provide six impacts and 22 J per second (12). The ME1000 allows the surgeon to use less force per individual strike but apply more force over-time providing a controlled method of impacting an acetabular shell, femoral broach, femoral stem or femoral head component. After the component is impacted, the impaction hand is removed, and the final position of the acetabular shell is confirmed with the JointPoint™ software. A Hole Eliminator is placed to prevent a fluid wave effect that may cause deterioration of bone behind the acetabular shell. The component is irrigated and inspected before placement of the liner.
To provide excellent visualization of the femur, several steps are taken. First, a hook attachment to the Judet-type orthopaedic table (Hana table, OSI, USA) is placed under the GT to help lift the proximal femur out of the wound as well as provide a firm backstop to support the femur during broaching and final component impaction (Figure 10). Next, traction is taken of the operative extremity and the leg spar holding the leg is extended to the floor and adducted below the contralateral extremity. This position of the femur delivers the osteotomized neck out of the incision as well as improves the trajectory for broach insertion, particularly for straighter stem designs.
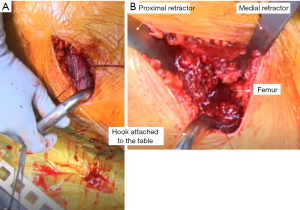
At this point, the femur is exposed, and the retractors can be placed. First, a Hohmann retractor is placed medially in the wound against the posterior femur between the cut femoral neck and the lesser trochanter. Next, a specialized GT retractor is placed in between the gluteus minimus and the superior capsule (Figure 10). This allows for the superior capsule to be sharply released from the superior and posterior neck. An Aquamantys® system (Medtronic, Minneapolis, MN, USA) device is used then cauterize any retinacular vessels, with a particular focus at the cut edges of the capsule and posterior to the femoral neck. This allows for visualization of the short external rotators. If there is any difficulty with the mobilization of the femur the piriformis and conjoint tendon can be sharply incised, in an oblique fashion from the tip of the GT towards the proximal femoral neck. The distally located obturator externus is always preserved.
Broaching and trialing of femoral component
In preparation to broach the femur, the specialized GT retractor is replaced by another specialized GT retractor that provides better exposure and room for the broaches. Any lateral neck is removed with either a Rongeur, box osteotome or both. Broaching is initiated with an opening broach. The senior author uses the Actis™ stem (DePuy Orthopaedics, Warsaw, IN, USA). The broaching is begun with the stem tip at the medial calcar. This allows the stem to follow the curve of the neck to the femur and when sized up appropriately will give the stem cortical contact against the calcar medially and the femoral cortex laterally. Once again, the ME1000 is utilized (Figure 11). After the opening broach, the senior author prefers to start with a stem that is approximately two sizes less than the preoperatively templated femoral stem. By using fewer broaches, there is less bone loss and expedites the broaching process. Broaching is performed until rotational stability is achieved, which is defined by the senior author as the lack of movement of the broach within the canal when subjected to a moderate rotational force.
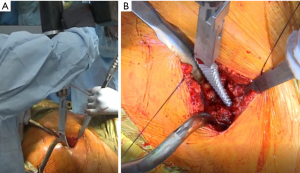
Once rotational stability is obtained, it is possible for the surgeon to begin trialing. When trialing, the senior surgeon uses fluoroscopy to determine the length and offset. It is possible to use fluoroscopy and print the images on clear acetate to be able to superimpose them, as described below, but the senior author prefers the ease and accuracy of computer navigation software such as JointPoint™ software or Radlink GPS™ software to perform the same function.
First, a PA of each hip is taken with the foot internally rotated approximately 15 degrees until maximum offset is achieved. It is important to move the fluoroscope in the same plane of an ideal PA fluoroscopic image to prevent any effects of magnification. If the contralateral side has pathology that prevents it from being used as a reference for length or offset, then the ipsilateral side can be used if the PA hip fluoroscopic image is taken prior to the femoral neck osteotomy. The contralateral image is then digitally “flipped” to appear as the operative side and the images are superimposed. The surgeon can then use a tonsil clamp to sterilely move the images on the touch screen up, down, left, and right but also rotate them. Landmarks on the pelvis such as the ilioischial line, iliopectineal line, teardrop and the lateral most corner of the remaining acetabulum can be used at the surgeon's discretion to line up the hips as close as possible. JointPoint™ software has the function where it is able to do so “automatically” by utilizing the assistant’s pre-marked points on each fluoroscopic image.
Next, the hip fluoroscopic images are rotated around the center of rotation of the newly implanted hip on the screen until the femurs are overlaid each other, as judged by the medial and lateral cortex and the GT. Once the fluoroscopic images are aligned, it is possible to accurately judge offset and length. JointPoint™ software includes a feature that gives the quantitative readout of length and offset in millimeters. With this information it possible to adjust the stem position, ball size or stem offset to make the length and offset to the desired length with precision. Additional broaching and/or stem, neck and ball trials can be changed and then confirmed using JointPoint™ software to achieve the exact length and offset with the final components.
Final femoral implant placement
After the process of sizing the stem, neck, and ball is complete, the remaining femoral neck is calcar planed to the level of the final planned broach. The final stem is impacted using the ME1000™ (Figure 12). Then the trunion is cleaned with normal saline and a laparotomy sponge before placing the femoral head. The senior author uses a Delta ceramic head due to its improved wear characteristics and avoidance of metal on metal contact to prevent the occurrence of trunionosis. The ME1000™ is used to impact the ceramic head, which due to its continuous impacting mechanism reaching 20 J of energy within less than 9 inches of swing distance, it delivers more energy per second. The cup is irrigated and inspected for any debris before final reduction. After reduction, final fluoroscopic images are taken to confirm length and offset as well as to evaluate the femur for any fracture.
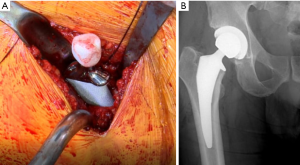
Closure
After copiously irrigating the wound with normal saline, the senior author uses liposomal bupivacaine (Exparel®; Pacira Pharmaceuticals, San Diego, CA, USA) to inject the capsule, pericapsular tissues, and skin for postoperative pain control. The two capsular sutures are tied together to repair the capsule. Next, the fascia overlying the TFL is repaired with a 1-0 running Vicryl suture. Lastly, the skin is closed with a subcutaneous running 2-0 Monocryl (Monocryl; Ethicon, Inc, Somerville, NJ, USA), a subcuticular 3-0 Monocryl and Prineo™ (Ethicon, Inc, Somerville, NJ, USA) tape.
Postoperative management
Patients are weight-bearing as tolerated postoperative day zero. They receive physical therapy in the recovery room and depending on the immediate postoperative course are discharged as early as the same day. There are no hip precautions limiting the range of motion. At the 3-month point, patients are able to resume all activities with exception of martial arts.
Discussion
The senior author began his building upon the work of Judet and Letournel in 1996. However, it was in 2003 when the first AA with the use of a specialized table course was provided, and after being disseminated to a broader audience, the AA has stepped into its modern phase of development. Since that time, the AA has progressed with new techniques and developments. Today, it is associated with shorter acute hospital stay and lower necessity of postoperative opiates compared to the posterior approach (6). In a cohort of 4,473 patients operated by the senior author, Barnett et al. reported an overall complication rate of 1.9% within 90 days after the procedure (13). At the same period and when compared with the posterior approach, the AA was found to be associated with lower mental but higher physical component summary scores, with similar complication rates between both approaches (14). Acceptable complications rates and improved functional outcomes were also reported by Bhandari et al. (11). Jewett and Collis reported a high incidence of complications with the AA performed on a traction table. Although the rate of trochanteric fractures was high, the authors found a good clinical recovery after adequate intraoperative treatment (15).
As any surgical technique, there is an associated learning curve, which can take up 100 cases (11). However, the senior author believes that once one begins to master the AA, a surgeon can consistently and reproducibly provide excellent surgical management for hip osteoarthritis with total hip arthroplasty. He encourages anyone interested to attend one of the AA courses where the fundamentals can be learned.
Key points
- Whenever taking fluoroscopic images, it is essential to be taking a true PA fluoroscopic imaging to be able to make accurate assessments of cup inclination and version in addition to femoral length and offset. The quality of the pelvic image is evaluated by looking at the symmetry of the obturator foramina, where the ilioischial line intersects with the teardrop, and where the sacrum is relative to the pubic symphysis (both in the medial-lateral direction as well as proximal to distal). Additionally, it is important to evaluate the preoperative supine/ AP radiograph to compare to the patient’s baseline image as well.
- Mobilization of the femur is crucial to having good exposure and therefore a safe and successful execution of the surgery. Dislocating the head “early” in the case may seem like an unnecessary step to some surgeons. However, it is crucial to allow a complete capsular release that facilitates exposure at multiple points later in the case.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Charlie C. Yang) for the series “Direct Anterior Approach (DAA) for Total Hip Arthroplasty (THA)” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: The series “Direct Anterior Approach (DAA) for Total Hip Arthroplasty (THA)” was commissioned by the editorial office without any funding or sponsorship. JM is Consultant for DePuy, Medtronic, Stryker, Invuity. Royalties—MizuhoOSI. Stockholder/stock options—Invuity; JointPoint; Medical Enterprise Distribution; Anterior Approach Instruments LLC; Radlink. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Judet J, Judet R. The use of an artificial femoral head for arthroplasty of the hip joint. J Bone Joint Surg Br 1950;32-B:166-73. [Crossref] [PubMed]
- Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop Relat Res 1970;7-21. [PubMed]
- Charnley J. Arthroplasty of the hip. A new operation. Lancet 1961;1:1129-32. [Crossref] [PubMed]
- Bergin PF, Doppelt JD, Kephart CJ, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am 2011;93:1392-8. [Crossref] [PubMed]
- Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res 2005;115-24. [Crossref] [PubMed]
- Cheng TE, Wallis JA, Taylor NF, et al. A Prospective Randomized Clinical Trial in Total Hip Arthroplasty-Comparing Early Results Between the Direct Anterior Approach and the Posterior Approach. J Arthroplasty 2017;32:883-90. [Crossref] [PubMed]
- Lahy J, Stevens J, McKenzie D, et al. The reliability of measuring acetabular component position on radiographs using everyday diagnostic imaging software. J Orthop Surg (Hong Kong) 2017;25:2309499017718953 [Crossref] [PubMed]
- Morvan A, Moreau S, Combourieu B, et al. Standing radiological analysis with a low-dose biplanar imaging system (EOS system) of the position of the components in total hip arthroplasty using an anterior approach: a cohort study of 102 patients. Bone Joint J 2016;98-B:326-33. [Crossref] [PubMed]
- Survey performed at the American Association of Hip and Knee Surgeons 2016 Annual Meeting, Washington, DC, USA.
- Kennon RE, Keggi JM, Wetmore RS, et al. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am 2003;85-A:39-48. [Crossref] [PubMed]
- Anterior Total Hip Arthroplasty Collaborative Investigators. Outcomes following the single-incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin North Am 2009;40:329-42. [Crossref] [PubMed]
- Available online: http://synthes.vo.llnwd.net/o16/LLNWMB8/US%20Mobile/Synthes%20North%20America/Product%20Support%20Materials/Brochures/DSUSJRC09172388_ME1000%20Surgical%20Impactor_Energy%20Analysis_DPS%20FINAL.pdf
[accessed 2/15/18] . - Barnett SL, Peters DJ, Hamilton WG, et al. Is the Anterior Approach Safe? Early Complication Rate Associated With 5090 Consecutive Primary Total Hip Arthroplasty Procedures Performed Using the Anterior Approach. J Arthroplasty 2016;31:2291-4. [Crossref] [PubMed]
- Graves SC, Dropkin BM, Keeney BJ, et al. Does Surgical Approach Affect Patient-reported Function After Primary THA? Clin Orthop Relat Res 2016;474:971-81. [Crossref] [PubMed]
- Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res 2011;469:503-7. [Crossref] [PubMed]
Cite this article as: Tatka J, Fagotti L, Matta J. Anterior approach total hip replacement (THA) with a specialized orthopedic table. Ann Joint 2018;3:42.


