
Contrast agents for optical diagnostics of early osteoarthritis
Introduction
Osteoarthritis (OA) is a joint disease accompanied by the thickening of articular cartilage and fibrillation of its surface (1). On molecular level it implies the gradual loss of glycosaminoglycans (GAGs) with subsequent destabilization of collagen network, deterioration of tissue mechanics and joint dysfunction (2). The standard methods of OA imaging are radiography (3,4), multimodal magnetic resonance tomography (MRT) (5,6), X-ray based computed tomography (CT) (3,6) and its high resolution analog µCT (6,7) (Table 1). The general limitation of the techniques is the inability to image the early stages of OA, or pre-OA, characterized by the presence of submicron defects (11) caused by GAGs depletion and collagen disorientation at superficial cartilage layer (12) (Figure 1). The alternative imaging approaches include ultrasound (6,13), atomic force microscopy (11,14), photoacoustic imaging (9) and a family of optical visualizing methods (10). Some of the new developing approaches, such as plasma-based biochemical test (15), are based on detection of specific blood biomarkers.

Full table

Optical coherent tomography (OCT) possesses high resolution cross-sectional imaging of cartilage up to several microns (10,16) and gives information about disorders of surface collagen orientation (10) which makes it prospective for the diagnostics of the early OA. However, it analyzes only the upper tissue layers (1–2 mm) and is still more invasive than MRT because in some cases the OCT tool is injected into the joint space during arthroscopy or intravenous (17). Moreover, the penetration depth of the probing beam and therefore the radius of diagnosis of OCT usually do not exceed 1–2 mm (16) due to the high scattering of light in cartilage (18,19).
An option to increase the sensitivity of optical methods towards evaluation of the early cartilage defects is the introduction of contrast agents capable to bind selectively to the altered sites of the tissue surface, e.g., submicron defects, disordered collagen, charged fragments of macromolecules, altered cells, reactive oxygen species, etc. Such molecules may serve as absorbers of probing radiation or as fluorescent labels emitting light after excitation by radiation with a certain wavelength. Further, the integration of sensitive optical diagnostics with targeted therapy of the early OA seems reasonable when the selected contrast agent enables to selectively absorb therapeutic laser radiation, or to deliver the drug directly to the area of damage. The present study is focused on the recent advances in developing of such materials for the optical diagnostics and treatment of cartilage.
Near infrared (NIR) fluorescent probes
Laser-activated NIR fluorescent imaging is the non-invasive diagnostic procedure allowing for the deeper tissue penetration due to the lower scattering of red or NIR light (650–900 nm) in tissues (20) and increased signal-to-noise ratio due to the lower tissue autofluorescence compared to the visible spectral range between 400 and 600 nm. A fluorescent probe usually consists of two parts: fluorescent dye (chromophore) and specific moiety serving for transport and proper binding to the area of interest. The working principle is to excite the dye with radiation of certain wavelength and to detect fluorescence with the sensitive camera.
There are several categories of the used probes (Table 2): conventional non-targeted dyes such as indocyanine green and methylene blue (20), specially designed targeted probes and so-called “smart” activity based probes with fluorescence depending on the enzymatic activity (20,26). Whereas most of the dyes and targeted probes are aimed at detection of local inflammation which is a reliable sigh of rheumatoid arthritis (21,22,27,28), some attempts are made to reveal sighs of early OA that is not accompanied by inflammation. Thus, the electrostatic interaction of positively charged fluorophores with anionic proteoglycans enables to visualize GAGs distribution in the same way as contrast enhanced CT diagnostics, being, however, the non-ionizing procedure (23).

Full table
Another approach is based on the monitoring of cartilage metalloprotease (MMP) activity known to enhance collagen degradation already at early stages of OA: the dual imaging method was recently presented allowing to reveal simultaneously the MMP activity along with collagen II accumulation within the same joint using the respective set of optical filters (24). Such a combination of two signals increases the reliability of determination of degenerated sites, though it still can only complement, not replace, the conventional diagnostics. Apoptotic cartilage chondrocytes also were detected with fluorescent-labeled apoptosis-targeting peptide-1 binding to histone H1 on the surface of apoptotic and necrotic cells (25). In mice model the authors showed that the distribution of apoptotic cells differs for normal and surgically destabilized cartilage already in 2 weeks after the surgery. Further, the peptide was modified by coupling with NIR dye into a polyethylene glycol polymer (29).
Therefore, NIR fluorescent imaging represents a sensitive, non-ionizing and comparatively rapid diagnostic tool to reveal early signs of OA. However, there are some noticeable drawbacks associated with the lack of information about accumulation and elution rates, selectivity, toxicity and long-term effects of the modified probes on tissues and organs. A complex study is needed towards the possibility of clinical applications. Furthermore, only easily accessible joint cartilages, e.g., finger or wrist joints, can be effectively non-invasive diagnosed using the methodology: the light scattering in covering soft tissues layers still possesses a limitation for the accurate analysis of the harder-to-reach areas such as the knee or hip joints.
It is also necessary to clarify some aspects concerning the safety of exposure to NIR radiation of the above mentioned techniques. The NIR radiation dissipates in cartilage due to the scattering and weak absorption by interstitial water, while matrix and cell components much less absorb NIR light (19). The range between 700 and 1,350 nm is the so-called optical or therapeutic window, where the light penetrates deeper into biological tissues. Absorbed NIR radiation transforms to vibrational transitions of the molecules thus increasing the tissue temperature. The power of the used diagnostic probes (<<1 W/cm2) and short-term application usually do not lead to a noticeable increase in tissue temperature. Thus, no thermal induced alterations have an opportunity to occur. In comparison to ionizing X-ray or UV radiation inducing electronic transitions in molecules and formation of free radicals NIR radiation does not directly affect the tissue organics. However, there are described effects caused by repetitive long-term exposure of NIR light. First of all, they are presented by skin erythematous or hyperpigmented dermatoses (30). Some studies noted the NIR-induced upregulation of matrix metalloproteases and subsequent oxidative stress in tissues at enormous dosages of irradiation (up to 2,000 mW/cm2). The power density of the commercial NIR imaging devices usually does not exceed 10–15 mW/cm2 (31) which is comparable to the part of NIR radiation produced by natural sunlight [20 mW/cm2 (30)]. Therefore, the long-term effects of such irradiation are negligible in comparison to the possible toxicity of the used agents that has to be investigated in detail in each case.
Nanoparticles (NPs)
NPs have long attracted the interest of researchers as potential tool for imaging and phototherapy of blood vessels, lymph nodes, tumors, inflammation or for the routine treatment monitoring (32,33). In theranostics, when diagnostic procedure is combined with therapy, NPs may serve as effective drug carriers or absorbing additives (for light therapy). Metallic NPs have some advantages compared to the fluorescent dyes due to the higher photostability and ability to absorb in the near and middle infrared spectral ranges where biotissues are more transparent (19). Yet only a few metallic NPs demonstrate low toxicity and are approved for clinical practice, such as iron oxides, e.g., magnetite, maghemite, and their biopolymer functionalized modifications (34).
Polymeric NPs present another kind of nanomaterials perspective as contrast enhancers for the optical imaging of biotissues as they are biocompatible and easily functionalized (33).
Let us consider the possibilities of using these types of NPs for early diagnosis of OA.
Metallic NPs
While fluorescent probes are used for selective binding to target molecules on the surface or in the tissue matrix, NPs can physically absorb on cartilage surface and diffuse into matrix. Cartilage is avascular tissue with liquid circulation through nanosized pores. The average size of the pores in healthy hyaline-type cartilage is 10–15 nm (35,36). Interestingly, pore size in healthy hyaline cartilage was estimated to be around 15 nm by analyzing the size of impregnated magnetite NPs (35). Subsequent independent measurements using NaCl thermoporometry revealed the pores of 11–14 nm (36). Thus, impregnation of NPs can provide reliable information about the cartilage nano-size porous structure. When NPs were introduced to cartilage with laser-induced small defects, imitating the early OA, the average size of observed impregnated NPs and their agglomerates increased to 50–100 nm (35) and in pericellular matrix (around chondrocytes) to 230 nm (37). So it was assumed that the destruction of the cartilaginous tissue occurs primarily in the areas adjacent to cartilage cells and containing a comparatively high concentration of proteoglycans (37).
The ability of magnetite NPs to absorb on articular cartilage defects allowing its optical detection was verified in routine ex vivo experiment. The fresh joint cartilage of the cow femoral epiphyseal bone obtained from the local meat plant in 1 hour after the slaughter was tested as shown in Figure 2A,B. Simulation of traumatic articular cartilage defects was performed.
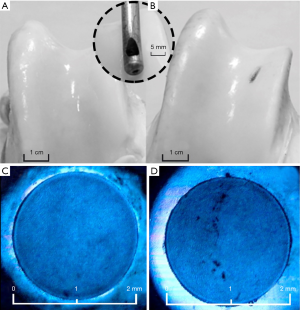
The two groups of traumatic defects were carried out. For the first group damage was applied to a half of the cartilage depth (0.2–0.3 mm) using a special instrument shown in the inset (Figure 2). A cotton swab soaked in an aqueous solution of magnetite NPs obtained as described in (35) at a concentration of 1.0 mg/mL was applied to the place of the macrodefect that was not visible with ordinary illumination. After 15 min of immersion with NPs the swab was removed and the treated site was washed with saline (0.9% NaCl) three times.
For the second group microdamages were applied on the surface of articular cartilage. Firstly, cylindrical cartilage samples 2 mm in diameter and 1 mm thick were cut from the bone epiphysis (Figure 2C,D). Microdamages in the form of scratches of 0.01–0.05 mm in depth were applied mechanically using a sharp needle. Then drops of the same solution of magnetite NPs were deposited on the damaged and intact sample, allow for 15 min to diffuse into the tissue and washed three times with saline. The samples were placed on a cover slip and examined with light microscope with a tenfold increase under the blue light emitting diode.
The one can see form Figure 2 that large defects can be easily visualized with the use of magnetite NPs dispersion. On the control sample the damaged area did not differ in color from the surrounding tissue (Figure 2A). Application of magnetite NPs on the damaged cartilage surface made it possible to visualize the defect by increasing its contrast in optical spectral range (Figure 2B). The contrast enhancing blue light manifestation of small defects with NPs is shown in Figure 2B,C.
The mechanism of anomalous adhesion of NPs to the damaged joint surface remains unclear. Nevertheless, the interaction of NPs with nanoscale cartilage components—collagen and proteoglycan aggregates can be considered within the framework of the Derjaguin-Landau-Verwey-Overbeek (DLVO) theory (38,39). According to the theory, describing colloidal systems, the attractive forces between two particles depend on the distance between them, and can change the sign (repulsion) at a distance comparable to the dimensions of the molecules. The energy of repulsion acting at small distances depends on the square zeta potential which increases with depth of the articular cartilage [according to zonality of the distribution of proteoglycans and a fixed negative charge (40)]. As a result of zeta effect of the proteoglycan potential, the stability of the colloidal solution of NPs is violated and, as a result, agglomeration occurs near the defect. The electrostatic interaction of particles almost does not occur on the joint surface of unaffected cartilage. Therefore, the adhesion of NPs to the undamaged surface is negligible.
It can be said that a defective structure has an increased energy (compared to an equilibrium intact structure), and the interaction of NPs with a defect reduces its energy, so the NPs predominantly absorb within the damaged zone.
Taking into account the noticeable thermal effect of magnetite NPs when cartilage is exposed to laser irradiation with the wavelength 1,560 nm (41), the ability of the NPs to adhere on the defects can be combined with the newly developing technology of laser-assisted OA healing (42) which is based on the thermomechanical NIR laser activation of the chondrocytes repair capacity. The abnormal concentration of absorbing NPs within submicrodefects enables to obtain the needed thermal effect locally at the damaged sites avoiding the heating of surroundings and deeper tissue layers. Moreover, the interstitial transport of magnetic NPs can be controlled with application of the external magnetic field.
Additional functionalization of magnetite NPs will enable their detection not only by means of MRT, but also by optical methods. For example, optical visualization was successfully performed for Rhodamine B labeled magnetite NPs with the mean size of 150 nm (43) which is close to that used for the impregnation into early cartilage defects (37). Multimodal MRT and NIR imaging can be realized when magnetic iron NPs are combined with gold half-shells (44). The plasmon resonance band of such multicomponent particles depends on structure, i.e., gold layering, and can be tuned within the optical transparency window of 650–900 nm or farther into IR region. Meanwhile magnetic properties of iron containing particles enable the MRT tracking of the probe (44). Silver NPs also are considered as potential contrast agents for tissue imaging due to their size tuned plasmon resonance wavelength that can be positioned in NIR region and enable deeper OCT scanning (45). However, as far as noble metallic NPs are concerned a question of their cytotoxicity usually arises.
Despite the aforementioned achievements, the clinical use of NPs for the early diagnosis of OA has a number of limitations remaining the task of future research. In preclinical research or combined with laser healing procedure the NPs solution may be applied directly to the surface of the opened joint. However, in case of the non-invasive diagnostics, e.g. OCT, there is the actual problem of the targeted delivery and accumulation of the NPs within the joint. Additionally, the quantitative methods of control of NPs concentration within the tissue should be optimized.
Polymeric NPs
Semiconducting polymeric NPs have a wide range of potential applications in imaging and therapy of different tissues (33). In some cases these NPs serve as moiety of fluorescent probes discussed in previous section designed for targeted binding to inflammation sites, e.g. for detection of reactive oxygen species (33). What is more, incorporation of the selectively reactive substrates directly into the composition of the NPs allows to visualize the variety of species, such as hydrogen peroxide H2O2, superoxide anion (O2−), reactive oxygen anions ONOO- and ClO− and others, or to monitor the interstitial pH level (33). Some of the modalities are based on chemiluminescent signal when no external excitation is needed. The dual optical and MRT modalities are also investigated for the conjugated polymeric NPs containing polyfluorene-based part for optical imaging and nitroxide stable free organic radical for MRT contrast (46).
Similar to the approaches developing for metallic NPs, targeted delivery of polymeric NPs is often combined with therapy aimed at slowing down or eliminating the symptoms of early OA.
Since cartilage is a dense elastic nano-porous material the drug diffusion from synovium and external vasculature to its matrix is difficult. As a consequence, the family of cartilage targeted nanodrugs is being developed (47). For example, simultaneous delivery of anti-inflammatory diclofenac and chondrogenic activator kartogenin with the use of polymeric oligosaccharide-based nanospheres to the damaged joint was demonstrated in animal model (48). The drug release was thermosensitive, achieved by 10 min application of cryotherapy (5 °C). The fluorescent labelling of nanospheres allowed to monitor their accumulation within joint by means of bioluminescence scanning system.
Another strategy of early OA treatment is based on inhibiting the expression of catabolic proteins using polymeric NPs as carrier of small-interfering RNA (49).
Though polymeric NPs do not contain heavy metals with potential high cytotoxicity, their biodistribution, clearance and long-term effects should be analyzed in detail before clinical applications.
Summing up, it should be noted that each considered NP approach has its own advantages and limitations. Thus, metallic and metallic oxide NPs can be relatively easily synthesized and functionalized, but they possess strong agglomeration in aqueous dispersions, which makes it difficult to obtain their concentrated solutions. Polymeric NPs are practically free of this drawback, however, they are usually difficult to produce requiring multistage organic synthesis with strict control of the conditions. A great advantage of iron based NPs is that they are FDA approved for medical use. Iron is involved in normal human metabolism, therefore its long-term effect is more likely to be neutral. Additionally, magnetic properties of iron enable the magnetically controlled introduction and removal of the particles as well as MRT monitoring of their transport, meanwhile the magnetic attraction of the particles enhances their agglomeration. Semiconducting polymeric NPs are considered to be biologically inert, although the long-term effects of their accumulated dosages are still poorly investigated that complicates their clinical application at least for the next several years. The targeted delivery of metallic NPs to early tissue defects is possible due to their anomalous adsorption on defects, while polymeric NPs should have functional groups for selective binding in damaged areas. In optical diagnostics metallic NPs can increase the contrast by strong absorption of optical radiation, whereas polymeric fluorescent NPs work in a different way—they emit light after appropriate excitation by an external source. Therefore, different optical schemes of signal registration are appropriate for the each kind of NPs.
Conclusions
The optical imaging modalities have the potential to overcome the spatial limitations of conventionally used diagnostics and reveal the development of OA at the submicron level when the joint alterations caused by the disease are not irreversible. The application of contrast agents, such as fluorescent dyes, functionalized polymeric or metallic-based NPs can substantially improve the sensitivity of diagnostics, as well as identify specific symptoms (or stages) of OA, i.e., submicron defects, inflammation, enzyme activity, apoptotic cells, GAGs concentration, etc. The future progress in optical imaging of OA is connected with the deeper understanding of complex mechanism of the disease development with the emphasis on processes on cellular and molecular level.
Acknowledgments
Funding: The work was supported by basic budget financing 0026-2014-0202 Nº116022610026 (program of fundamental scientific research of state academies of sciences: IV Nº37 “Scientific fundamentals and applications of information technology in medicine”) and partially supported by the Russian Foundation for Basic Research grant Nº15-29-04810.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.03.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tummala S, Bay-Jensen AC, Karsdal MA, et al. Diagnosis of Osteoarthritis by Cartilage Surface Smoothness Quantified Automatically from Knee MRI. Cartilage 2011;2:50-9. [Crossref] [PubMed]
- Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect 2005;54:465-80. [PubMed]
- Mathiessen A, Cimmino MA, Hammer HB, et al. Imaging of osteoarthritis (OA): What is new? Best Pract Res Clin Rheumatol 2016;30:653-69. [Crossref] [PubMed]
- Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis 1957;16:494-502. [Crossref] [PubMed]
- Menashe L, Hirko K, Losina E, et al. The diagnostic performance of MRI in osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2012;20:13-21. [Crossref] [PubMed]
- Thote T, Moran S, Lin A, et al. Imaging methods for detection of joint degeneration. In: Morris P. editor. Biomedical Imaging. Woodhead Publishing, 2014:235-65.
- Das Neves Borges P, Forte AE, Vincent TL, et al. Rapid, automated imaging of mouse articular cartilage by microCT for early detection of osteoarthritis and finite element modelling of joint mechanics. Osteoarthritis Cartilage 2014;22:1419-28. [Crossref] [PubMed]
- Bansal GJ. Digital radiography. A comparison with modern conventional imaging. Postgrad Med J 2006;82:425-8. [Crossref] [PubMed]
- Sun Y, Jiang H, O’Neill BE. Photoacoustic imaging: an emerging optical modality in diagnostic and theranostic medicine. J Biosens Bioelectron 2011;2:108. [Crossref]
- Matcher SJ. What can biophotonics tell us about the 3D microstructure of articular cartilage? Quant Imaging Med Surg 2015;5:143-58. [PubMed]
- Stolz M, Gottardi R, Raiteri R, et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat Nanotechnol 2009;4:186-92. [Crossref] [PubMed]
- Panula HE, Hyttinen MM, Arokoski JP, et al. Articular cartilage superficial zone collagen birefringence reduced and cartilage thickness increased before surface fibrillation in experimental osteoarthritis. Ann Rheum Dis 1998;57:237-45. [Crossref] [PubMed]
- Ruta S, Reginato AM, Pineda C, et al. General applications of ultrasound in rheumatology: why we need it in our daily practice. J Clin Rheumatol 2015;21:133-43. [Crossref] [PubMed]
- Han B, Nia HT, Wang C, et al. AFM-Nanomechanical Test: An interdisciplinary tool that links the understanding of cartilage and meniscus biomechanics, osteoarthritis degeneration, and tissue engineering. ACS Biomater Sci Eng 2017;3:2033-49. [Crossref]
- Ahmed U, Anwar A, Savage RS, et al. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res Ther 2016;18:250. [Crossref] [PubMed]
- Chu CR, Williams AA, Coyle CH. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther 2012;14:212. [Crossref] [PubMed]
- Cernohorsky P, de Bruin DM, van Herk M, et al. In-situ imaging of articular cartilage of the first carpometacarpal joint using co-registered optical coherence tomography and computed tomography. J Biomed Opt 2012;17:060501 [Crossref] [PubMed]
- Tuchin VV. Tissue optics and photonics: light-tissue interaction. J Biomed Photonics Eng 2015;1:98-135. [Crossref]
- Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol 2013;58:R37. [Crossref] [PubMed]
- Slooter MD, Bierau K, Chan AB, et al. Near infrared fluorescence imaging for early detection, monitoring and improved intervention of diseases involving the joint. Connect Tissue Res 2015;56:153-60. [Crossref] [PubMed]
- Schäfer VS, Hartung W, Hoffstetter P. Quantitative assessment of synovitis in patients with rheumatoid arthritis using fluorescence optical imaging. Arthritis Res Ther 2013;15:R124. [Crossref] [PubMed]
- Yin C, Zhu H, Xie C, et al. Organic Nanoprobe Cocktails for Multilocal and Multicolor Fluorescence Imaging of Reactive Oxygen Species. Adv Funct Mater 2017;27:1700493 [Crossref]
- Hu X, Wang Q, Liu Y, et al. Optical imaging of articular cartilage degeneration using near-infrared dipicolylamine probes. Biomaterials 2014;35:7511-21. [Crossref] [PubMed]
- Cho H, Bhatti FU, Lee S, et al. In vivo dual fluorescence imaging to detect joint destruction. Artif Organs 2016;40:1009-13. [Crossref] [PubMed]
- Che X, Chi L, Park CY, et al. A novel method to detect articular chondrocyte death during early stages of osteoarthritis using a non-invasive ApoPep-1 probe. Arthritis Res Ther 2015;17:309. [Crossref] [PubMed]
- Schwenck J, Maier FC, Kneilling M, et al. Non-invasive In Vivo Fluorescence Optical Imaging of Inflammatory MMP Activity Using an Activatable Fluorescent Imaging Agent. J Vis Exp 2017; [Crossref] [PubMed]
- Fischer T, Ebert B, Voigt J, et al. Detection of rheumatoid arthritis using non-specific contrast enhanced fluorescence imaging. Acad Radiol 2010;17:375-81. [Crossref] [PubMed]
- Zhang S, Shao P, Ling X, et al. In vivo inflammation imaging using a CB2R-targeted near infrared fluorescent probe. Am J Nucl Med Mol Imaging 2015;5:246-58. [PubMed]
- Huang Y, Zhou J, Hakamivala A, et al. An optical probe for detecting chondrocyte apoptosis in response to mechanical injury. Sci Rep 2017;7:10906. [Crossref] [PubMed]
- Barolet D, Christiaens F, Hamblin MR. Infrared and skin: Friend or foe. J Photochem Photobiol B 2016;155:78-85. [Crossref] [PubMed]
- Marshall MV, Rasmussen JC, Tan IC, et al. Near-Infrared Fluorescence Imaging in Humans with Indocyanine Green: A Review and Update. Open Surg Oncol J 2010;2:12-25. [Crossref] [PubMed]
- Mody VV, Siwale R, Singh A, et al. Introduction to metallic nanoparticles. J Pharm Bioallied Sci 2010;2:282-9. [Crossref] [PubMed]
- Li J, Rao J, Pu K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials 2018;155:217-35. [Crossref] [PubMed]
- Bobo D, Robinson KJ, Islam J. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res 2016;33:2373-87. [Crossref] [PubMed]
- Soshnikova YM, Roman SG, Chebotareva NA, et al. Starch modified magnetite nanoparticles for impregnation into cartilage. J Nanopart Res 2013;15:2092. [Crossref]
- Majda D, Bhattarai A, Riikonen J, et al. New approach for determining cartilage pore size distribution: NaCl-thermoporometry. Micropor Mesopor Mat 2017;241:238-45. [Crossref]
- Soshnikova YM, Shekhter AB, Baum OI. Laser radiation effect on chondrocytes and intercellular matrix of costal and articular cartilage impregnated with magnetite nanoparticles. Lasers Surg Med 2015;47:243-51. [Crossref] [PubMed]
- Derjaguin B, Landau LD. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim URSS 1941;14:633-62.
- Verwey EJW, Overbeek JT, Nes K. Theory of the stability of lyophobic colloids: the interaction of sol particles having an electric double layer, Elsevier, 1948:205.
- Mow VC, Guo XE. Mechano-Electrochemical properties of articular cartilage: Their Inhomogeneities and Anisotropies. Annu Rev Biomed Eng 2002;4:175-209. [Crossref] [PubMed]
- Alexandrovskaya Y, Sadovnikov K, Sharov A, et al. Controlling the near-infrared transparency of costal cartilage by impregnation with clearing agents and magnetite nanoparticles. J Biophotonics 2018;11: [Crossref] [PubMed]
- Sobol E, Shekhter A, Guller A, et al. Laser-induced regeneration of cartilage. J Biomed Opt 2011;16:080902 [Crossref] [PubMed]
- Eghbali P, Fattahi H, Laurent S, et al. Fluorophore-tagged superparamagnetic iron oxide nanoparticles as bimodal contrast agents for MR/optical imaging. J Iran Chem Soc 2016;13:87-93. [Crossref]
- Kim HJ, Lee SM, Park KH. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials 2015;61:95-102. [Crossref] [PubMed]
- Mondal I, Raj S, Roy P, et al. Silver nanoparticles (AgNPs) as a contrast agent for imaging of animal tissue using swept-source optical coherence tomography (SSOCT). Laser Phys 2018;28:015601 [Crossref]
- Hou M, Lu X, Zhang Z, et al. Conjugated Polymer Containing Organic Radical for Optical/MR Dual-Modality Bioimaging. ACS Appl Mater Interfaces 2017;9:44316-23. [Crossref] [PubMed]
- Bottini M, Bhattacharya K, Fadeel B, et al. Nanodrugs to target articular cartilage: An emerging platform for osteoarthritis therapy. Nanomedicine 2016;12:255-68. [Crossref] [PubMed]
- Kang ML, Kim JE, Im GI. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater 2016;39:65-78. [Crossref] [PubMed]
- Pi Y, Zhang X, Shao Z. Intra-articular delivery of anti-Hif-2α siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther 2015;22:439-48. [Crossref] [PubMed]
Cite this article as: Alexandrovskaya YM, Omelchenko AI, Shekhter AB, Sobol EN. Contrast agents for optical diagnostics of early osteoarthritis. Ann Joint 2018;3:25.


