Surgical anatomy of the direct anterior approach for total hip arthroplasty
Introduction
Total hip arthroplasty (THA), first described in its modern form by Charnley in 1961has revolutionized the treatment of hip arthritis (1). A number of different approaches have been described to access the hip joint, including the direct anterior approach (DAA), which was well described approximately 100 years ago by Smith-Peterson (2) and more recently popularized (3,4), gaining renewed interest in the past few decades, owing to its muscle-sparing nature, employment of a true internervous interval, and favorable postoperative stability (5), among other purported advantages. Some data suggest less postoperative pain and shorter length of stay while maintaining reasonable operating times (6). Berend et al. (7) have outlined potential early outcome advantages associated with the DAA, including more likely discharge to home and higher hip scores in very early follow up. Other studies have reinforced excellent early outcomes (8). Radiographic studies have shown that muscle damage may be lower with the anterior approach as compared to the posterior approach, as quantified with magnetic resonance imaging (9). While the approach has been described as overall being safe and accessible (10), there are concerns over the technically challenging nature of the approach, with a noted learning curve (11). A number of characteristic complications have been described associated with the unique anatomy of this approach to THA (11-14). Herein we review the relevant anatomy, and cautions and dangers characteristically associated with the DAA.
DAA anatomic limitations and extensibility
A number of concerns exist in total joint arthroplasty done in the obese patient, and the DAA is no exception. Higher BMI patients have been shown to be at higher risks for increased operating time, bleeding risks and overall complication rates (15). Infection in particular has been shown to be higher in those with BMI >35 undergoing DAA THA (16). Furthermore, the Mayo Clinic registry data has shown wound complications to be higher in obese patients undergoing DAA, though still similar to those undergoing posterior THA (17).
One of the purported benefits of the posterior approach as compared to the DAA for THA is the extensile nature of the former. Ghijselings and colleagues have described (18), in a cadaveric model, the distal extension of the DAA, and the locations of neurovascular bundles in relation to the distal extent of the approach. They conclude that it is feasible to extend the DAA, and report a series of extension of the DAA using this “interbundle technique (19),” which could be employed to gain access to the femur, should cabling or other processes be required. Other reported extensions of the DAA (20) recommend exercising caution when employing, owing to potential devitalization of neurovascular structures supplying the quadriceps musculature, specifically with application of a cerclage wire passer.
Neurological considerations
While neurovascular injuries to major structures are rare during THA, specific risks must be considered with any approach, with particular attention paid to specific structures at risk with a given surgical approach and dissection. In general, motor nerve injuries are rare; sciatic nerve injury is more characteristic with posterior approaches, superior gluteal nerve injury associated with lateral approaches (21), and the DAA with femoral nerve injury, though very uncommon (22). Cadaveric studies have demonstrated that, in associated with acetabular retractors, nerve impingement is possible, and specifically that anterior retraction may harm the femoral nerve if contact with acetabular bone is not direct, or if the acetabular retractor migrates over time (23).
Owing to the proximity of the dissection used, the DAA has been associated with damage to the lateral femoral cutaneous nerve (LFCN) (3,12,24). While no motor function is lost with damage to the LFCN, it can be associated with burning pain, though rarely with any functional deficits reported (24). A cadaveric study has shown the location of the LFCN in reference to different anatomic landmarks, and may emerge either above or below the inguinal ligament (25). Reference to bony landmarks, and ratios of distances rather than absolute measurements themselves may be better guides to where the LFCN is, in the authors’ opinion. Patients with smaller measures of hip offset may be at higher risk of LFCN injury during the DAA as well (26), presumably due to the close proximity of the structures involved and smaller amount of area in between incision and the nerve.
Osseous considerations
While characteristically providing excellent access and visibility to the acetabulum, the femoral exposure and visualization can prove more problematic in the DAA for THA. Trochanteric fractures are relatively more common with this approach, and femoral perforations and calcar fractures are well reported as well (14). Retractors placed during femoral preparation may be associated with fractures of the greater trochanter, in one study, occurring nearly 30% of the time (27). In cases of smaller fragments, the clinical significant of greater trochanter fractures is unclear, but smaller trochanters appear to be at greater risk for fracture (27). More displaced or larger trochanter fragments may prove to be more clinically significant and pose considerable morbidity to the patient, especially if associated with abductor dysfunction or Trendelenburg gait.
De Geest (13) described a series of 300 DAA THA patients and noted at least three types of femur-related bony complications: three of which were greater trochanter fractures, two femoral perforations during preparation, and four calcar fractures. However, there were also five patients who were noticed postoperatively to have sustained periprosthetic fractures that were not apparent intraoperatively. They attribute these to have occurred occultly during the surgery. Berend et al. (28) reviewed nearly 3,000 DAA THA, of which 26 sustained periprosthetic femur fractures. The only identified risk factor was increased age in the female patient, for whom they recommend exercising caution, different surgical approach or implant design.
Muscular anatomic considerations
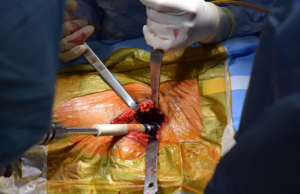
A purported benefit of the DAA for THA is the internervous nature of the approach and preservation of muscular attachments (Figure 1). However, muscle damage still occurs during the approach (29), and to the extent possible, should be limited. Appropriate function of hip abductor musculature is necessary for good clinical outcomes after THA; abductor deficiency after THA presents a challenging clinical scenario with a number of described techniques to treat (30-32). Radiographic studies have demonstrated that there can be fatty infiltration of gluteus medius and minimus muscles indicating denervation damage after THA (33,34). While appropriate abductor function is necessary after THA, damage, as evaluated by MRI, to the gluteus minimus is of less clinical importance than is harm to the gluteus medius (33). While at less risk than during lateral approaches to the hip, the hip abductors must be protected during the DAA, both during the approach as well as bony preparation.
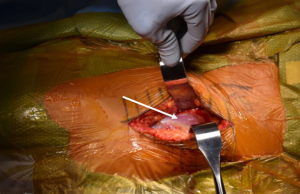
Capsular release and femoral elevation are performed as prerequisites for femoral preparation during the DAA, as appropriate visualization is not possible without doing so. The short external rotators are ideally preserved, even with posterior capsular release for femoral elevation. Cadaveric studies have mapped the short external rotators and conjoined tendon as extending to the anterosuperior portion of the greater trochanter (35). Therefore, during capsular release for exposure and elevation prior to femoral preparation, knowledge of the location of these musculotendinous structures must be known in order to maintain their integrity and preserve the stability they offer after THA.
Technique-specific anatomic considerations
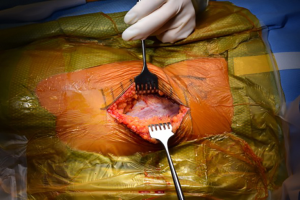
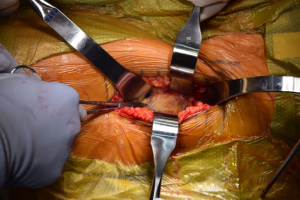
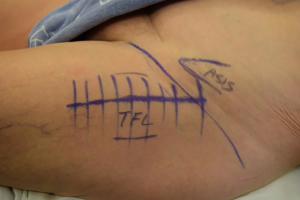
The incision for the DAA is characteristically based off the anatomic location of the anterior superior iliac spine and the greater trochanter (Figure 2) (4). So-called “bikini” modifications of the incision have been described as variations of the technique (36,37). After the fascia over the tensor fascia lata (TFL) is incised (Figure 3), the interval between the TFL and the rectus femoris is bluntly developed. It is at this point that the ascending branches of the lateral femoral circumflex artery are encountered (20) (Figure 4). Once ligated and divided, the pericapsular region is accessible, and is overlain by the reflected head of the rectus femoris. Retractors are placed on either side of the femoral neck and the capsule is exposed by medial reflection and retraction of the rectus femoris. After incising the capsule, the bony femoral neck is accessible for osteotomy (Figure 5).
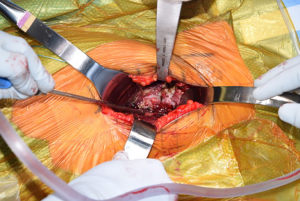
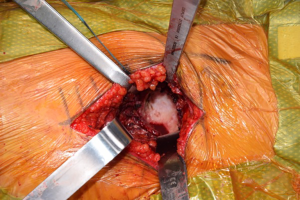
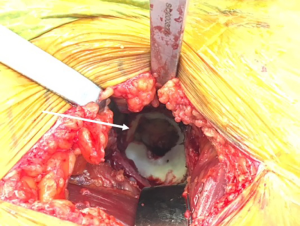
Once the femoral neck cut is made and the head removed, the acetabulum is exposed (Figure 6). Much has been written on the relationship of the transverse acetabular ligament (TAL), and its relationship to orientation of the acetabulum preparation and component placement (Figure 7). By identifying the TAL, it may be used as a guide for version and therefore cup position (38-40). However, some authors have noted that the TAL is difficult to accurately identify for positioning purposes (41). Debate exists regarding its use in intraoperative guidance of acetabular positioning in anatomic variations, including dysplastic acetabulae (42,43). Regardless of its utility in guiding acetabular positioning, along with the cotyloid fossa, the TAL may be used as an anatomic landmark to avoid over-medialization of the acetabular reamers (44). During acetabular reaming, the TFL, rectus femoris, and femoral neck cut may all be the cause of anatomic structures that can deflect acetabular reamers, so exposure must be adequate (Figure 8).
Following completion of the acetabular preparation and cup placement, femoral preparation is undertaken. It is at this point that posterior capsular releases are performed both for appropriate retractor placement as well as for anterior translation of the proximal femur so that the canal can be accessed for bony preparation and broach and implant insertion (4,45). This portion of the operation may be especially technically demanding and dependent on appropriate technique. In order to present the cut face of the femoral neck and allow entrance to the femoral canal, the femur is extended, externally rotated, adducted, and the proximal femur elevated for optimal anatomic positioning. During femoral broaching, a retractor is often placed on the medial aspect of the cut femoral neck, and the authors recommend exercising caution not to damage the iliopsoas tendon which is inferior to this, at the level of the lesser trochanter. With the sum of these anatomic considerations, the components can be safely and reproducibly placed in the DAA.
Conclusions
The DAA for THA is gaining in popularity due to a number of potential benefits the approach offers. However, there are a number of noted complications that are presented by the unique anatomic considerations and challenges associated with this approach. With a thorough knowledge of the involved anatomy and application of sound surgical technique at all points of the operation, the DAA may be safely employed to accomplish THA.
Acknowledgments
The authors would like to acknowledge Roseann Johnson, for her assistance in manuscript preparation and submission.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Direct Anterior Approach (DAA) for Total Hip Arthroplasty (THA)”. The article has undergone external peer review.
Conflicts of Interest: The series “Direct Anterior Approach (DAA) for Total Hip Arthroplasty (THA)” was commissioned by the editorial office without any funding or sponsorship. CCY served as the unpaid Guest Editor of the series and serves as an unpaid associate editor of Annals of Joint from Dec 2016 to Dec 2018. CCY is a paid presenter or speaker for Zimmer Biomet, Medtronic, and DePuy, a Johnson & Johnson company, a paid consultant for DePuy, and receives research support from DePuy. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Charnley J. Arthroplasty of the hip. A new operation. Lancet 1961;1:1129-32. [Crossref] [PubMed]
- Smith-Peterson MN. A New Supra-Articular Subperiosteal Approach to the Hip. Am J Orthop Surg 1917;15:592-5.
- Light TR, Keggi KJ. Anterior approach to hip arthroplasty. Clin Orthop Relat Res 1980;255-60. [PubMed]
- Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res 2005;115-24. [Crossref] [PubMed]
- Siguier T, Siguier M, Brumpt B. Mini-incision anterior approach does not increase dislocation rate: a study of 1037 total hip replacements. Clin Orthop Relat Res 2004;164-73. [Crossref] [PubMed]
- Alecci V, Valente M, Crucil M, et al. Comparison of primary total hip replacements performed with a direct anterior approach versus the standard lateral approach: perioperative findings. J Orthop Traumatol 2011;12:123-9. [Crossref] [PubMed]
- Berend KR, Lombardi AV, Seng BE, et al. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am 2009;91:107-20. [Crossref] [PubMed]
- Nakata K, Nishikawa M, Yamamoto K, et al. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty 2009;24:698-704. [Crossref] [PubMed]
- Agten CA, Sutter R, Dora C, et al. imaging of soft tissue alterations after total hip arthroplasty: comparison of classic surgical approaches. Eur Radiol 2017;27:1312-21. [Crossref] [PubMed]
- Barnett SL, Peters DJ, Hamilton WG, et al. Is the Anterior Approach Safe? Early Complication Rate Associated With 5090 Consecutive Primary Total Hip Arthroplasty Procedures Performed Using the Anterior Approach. J Arthroplasty 2016;31:2291-4. [Crossref] [PubMed]
- Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics 2008;31. [PubMed]
- De Geest T, Fennema P, Lenaerts G, et al. Adverse effects associated with the direct anterior approach for total hip arthroplasty: a Bayesian meta-analysis. Arch Orthop Trauma Surg 2015;135:1183-92. [Crossref] [PubMed]
- De Geest T, Vansintjan P, De Loore G. Direct anterior total hip arthroplasty: complications and early outcome in a series of 300 cases. Acta Orthop Belg 2013;79:166-73. [PubMed]
- Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res 2011;469:503-7. [Crossref] [PubMed]
- Sang W, Zhu L, Ma J, et al. The Influence of Body Mass Index and Hip Anatomy on Direct Anterior Approach Total Hip Replacement. Med Princ Pract 2016;25:555-60. [Crossref] [PubMed]
- Purcell RL, Parks NL, Gargiulo JM, et al. Severely Obese Patients Have a Higher Risk of Infection After Direct Anterior Approach Total Hip Arthroplasty. J Arthroplasty 2016;31:162-5. [Crossref] [PubMed]
- Watts CD, Houdek MT, Wagner ER, et al. High Risk of Wound Complications Following Direct Anterior Total Hip Arthroplasty in Obese Patients. J Arthroplasty 2015;30:2296-8. [Crossref] [PubMed]
- Ghijselings SG, Driesen R, Simon JP, et al. Distal Extension of the Direct Anterior Approach to the Hip: A Cadaveric Feasibility Study. J Arthroplasty 2017;32:300-3. [Crossref] [PubMed]
- Ghijselings SG, Driesen R, Simon JP, et al. Distal Extension of the Anterior Approach to the Hip Using the Femoral Interbundle Technique: Surgical Technique and Case Series. J Arthroplasty 2017;32:2186-90. [Crossref] [PubMed]
- Grob K, Monahan R, Gilbey H, et al. Distal extension of the direct anterior approach to the hip poses risk to neurovascular structures: an anatomical study. J Bone Joint Surg Am 2015;97:126-32. [Crossref] [PubMed]
- Basarir K, Ozsoy MH, Erdemli B, et al. The safe distance for the superior gluteal nerve in direct lateral approach to the hip and its relation with the femoral length: a cadaver study. Arch Orthop Trauma Surg 2008;128:645-50. [Crossref] [PubMed]
- Yang IH. Neurovascular Injury in Hip Arthroplasty. Hip Pelvis 2014;26:74-8. [Crossref] [PubMed]
- McConaghie FA, Payne AP, Kinninmonth AW. The role of retraction in direct nerve injury in total hip replacement: an anatomical study. Bone Joint Res 2014;3:212-6. [Crossref] [PubMed]
- Goulding K, Beaulé PE, Kim PR, et al. Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res 2010;468:2397-404. [Crossref] [PubMed]
- Üzel M, Akkin SM, Tanyeli E, et al. Relationships of the lateral femoral cutaneous nerve to bony landmarks. Clin Orthop Relat Res 2011;469:2605-11. [Crossref] [PubMed]
- Ozaki Y, Homma Y, Sano K, et al. Small femoral offset is a risk factor for lateral femoral cutaneous nerve injury during total hip arthroplasty using a direct anterior approach. Orthop Traumatol Surg Res 2016;102:1043-7. [Crossref] [PubMed]
- Homma Y, Baba T, Ochi H, et al. Greater trochanter chip fractures in the direct anterior approach for total hip arthroplasty. Eur J Orthop Surg Traumatol 2016;26:605-11. [Crossref] [PubMed]
- Berend KR, Mirza AJ, Morris MJ, et al. Risk of Periprosthetic Fractures With Direct Anterior Primary Total Hip Arthroplasty. J Arthroplasty 2016;31:2295-8. [Crossref] [PubMed]
- Meneghini RM, Pagnano MW, Trousdale RT, et al. Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin Orthop Relat Res 2006;293-8. [Crossref] [PubMed]
- Whiteside LA, Nayfeh T, Katerberg BJ. Gluteus Maximus Flap Transfer for Greater Trochanter Reconstruction in Revision THA. Clin Orthop Relat Res 2006;203-10. [Crossref] [PubMed]
- Rao BM, Kamal TT, Vafaye J, et al. Surgical repair of hip abductors. A new technique using Graft Jacket allograft acellular human dermal matrix. Int Orthop 2012;36:2049-53. [Crossref] [PubMed]
- Fehm MN, Huddleston JI, Burke DW, et al. Repair of a deficient abductor mechanism with Achilles tendon allograft after total hip replacement. J Bone Joint Surg Am 2010;92:2305-11. [Crossref] [PubMed]
- Müller M, Tohtz S, Winkler T, et al. MRI findings of gluteus minimus muscle damage in primary total hip arthroplasty and the influence on clinical outcome. Arch Orthop Trauma Surg 2010;130:927-35. [Crossref] [PubMed]
- Pfirrmann CWA, Notzli HP, Dora C, et al. Abductor tendons and muscles assessed at MR imaging after total hip arthroplasty in asymptomatic and symptomatic patients. Radiology 2005;235:969-76. [Crossref] [PubMed]
- Ito Y, Matsushita I, Watanabe H, et al. Anatomic Mapping of Short External Rotators Shows the Limit of Their Preservation During Total Hip Arthroplasty. Clin Orthop Relat Res 2012;470:1690-5. [Crossref] [PubMed]
- Lanting BA, Hartley KC, Raffoul AJ, et al. Bikini versus traditional incision direct anterior approach: is there any difference in soft tissue damage? Hip Int 2017;27:397-400. [Crossref] [PubMed]
- Leunig M, Faas M, von Knoch F, et al. Skin Crease “Bikini” Incision for Anterior Approach Total Hip Arthroplasty: Surgical Technique and Preliminary Results. Clin Orthop Relat Res 2013;471:2245-52. [Crossref] [PubMed]
- Hiddema WB, van der Merwe JF, van der Merwe W. The Transverse Acetabular Ligament as an Intraoperative Guide to Cup Abduction. J Arthroplasty 2016;31:1609-13. [Crossref] [PubMed]
- Salal MH. Transverse Acetabular Ligament as an Anatomical Landmark for Intraoperative Cup Anteversion in Primary Total Hip Replacement. J Coll Physicians Surg Pak 2017;27:642-4. [PubMed]
- Idrissi ME, Elibrahimi A, Shimi M, et al. Acetabular component orientation in total hip arthroplasty: the role of acetabular transverse ligament. Acta Ortop Bras 2016;24:267-9. [Crossref] [PubMed]
- Jain S, Aderinto J, Bobak P. The role of the transverse acetabular ligament in total hip arthroplasty. Acta Orthop Belg 2013;79:135-40. [PubMed]
- Abe H, Sakai T, Hamasaki T, et al. Is the transverse acetabular ligament a reliable cup orientation guide? Acta Orthopaedica 2012;83:474-80. [Crossref] [PubMed]
- Miyoshi H, Mikami H, Oba K, et al. Anteversion of the acetabular component aligned with the transverse acetabular ligament in total hip arthroplasty. J Arthroplasty 2012;27:916-22. [Crossref] [PubMed]
- Harris MJ, Tam J, Fineberg SJ, et al. Quantifying the Relationship Between the Transverse Acetabular Ligament and the Radiographic Teardrop. J Arthroplasty 2017;32:296-9. [Crossref] [PubMed]
- Nogler M, Krismer M, Hozack WJ, et al. A double offset broach handle for preparation of the femoral cavity in minimally invasive direct anterior total hip arthroplasty. J Arthroplasty 2006;21:1206-8. [Crossref] [PubMed]
Cite this article as: Holst DC, Yang CC. Surgical anatomy of the direct anterior approach for total hip arthroplasty. Ann Joint 2018;3:23.