Pain and the unstable knee
Introduction
Stability is crucial for proper knee function. In this regard, multiple anatomic structures provide primary and secondary stabilizing forces to the knee. Primary stabilizers include the anterior cruciate ligament, posterior cruciate ligament (PCL), medial collateral ligament (MCL) and the posteromedial (PM) complex, and lateral collateral ligament and posterolateral corner (PLC). Secondary stabilizers include the menisci, patella and patella tendon, muscles, as well as cartilage and bony anatomy. Significant pain can occur with injury to the primary stabilizers, thereby leading to an unstable knee. Stress is then commonly transferred to the secondary structures, potentially causing further injury and degeneration, which can result in significant sources of knee pain and dysfunction.
Medial knee injury
Anatomy and biomechanics
The medial collateral ligament (MCL) is a static valgus stabilizer and is commonly injured when a valgus and external rotation force is applied to the knee. More severe injury usually is caused by a direct blow to the lateral knee, compared with an often less severe non-contact injury. The MCL is comprised of a superficial and deep section. The superficial MCL originates from the medial epicondyle and inserts distally on the posteromedial aspect of the tibia, approximately 5 to 6 cm from the joint. The deep component is composed of the meniscotibial and meniscofemoral ligaments (MFLs), which merge with the medial capsule of the knee. MCL ruptures occur most commonly from the femoral insertion. This is also where the MCL has the greatest potential to heal non-surgically. MCL tears also commonly occur when the anterior cruciate ligament (ACL) and/or menisci are injured (1-3).
Physical exam and grading
Injury to the MCL should be suspected when a patient reports a valgus producing injury to the knee. On examination, patients are typically tender with palpation medially along the course of the MCL at the medial epicondyle or proximal tibia. Given that the MCL is the principal restraint to valgus stress at 30° of flexion, the knee should therefore be examined in this way to assess medial joint gapping. MCL injury grading corresponds to: grade I: <5 mm of joint line opening, grade II: 5–10 mm of joint line opening, and grade III: >10 mm of joint line opening. With experience, severity of injury to the MCL can be assessed based on the feeling of the end point. A first-degree lesion has significant palpation tenderness along the MCL without laxity. A second-degree injury demonstrates valgus laxity, but has a firm end point. A third-degree injury has substantial laxity without an end point. With third-degree injuries to the MCL, the examiner must suspect a multiple ligament knee injury, as secondary valgus stabilizers, such as the ACL and posterior cruciate ligament (PCL), may have been injured concomitantly.
Treatment
Grading of medial sided knee injury determines the treatment. Grade I MCL injuries recover well with avoidance of sport and aggravating activities, nonsteroidal anti-inflammatories (NSAIDs), as well as early physical therapy, with return to play typically expected after 5 to 7 days. For isolated grade II and III MCL injuries, the same treatment can be recommended, but with the addition of a hinged knee brace (3). Return to sport is characteristically after 2 to 4 weeks for grade II MCL injuries, while grade III injuries typically take 4 to 8 weeks (4). Prophylactic MCL functional bracing has been shown to prevent injury with American football lineman and is recommended (5). Surgery is indicated in multiligamentous injuries, as well as in grade III injuries that have persistent instability after a trial of non-operative management (3). MCL repair is efficacious with acute MCL avulsions utilizing suture anchor fixation or a soft tissue washer and screw construct. Reconstruction of the MCL is performed if the ligament is deemed not repairable, or with chronic instability. Commonly used grafts are hamstring autograft, as well as tibialis anterior or Achilles allograft (3). Concomitant injuries to the menisci, cartilage and other knee structures can be a substantial source of pain. Continued knee instability can also potentiate pain and may lead to abnormal wear and more chronic sources of knee pain.
Lateral and posterolateral knee injury
Anatomy and biomechanics
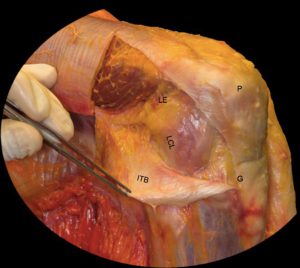
Structures of the lateral knee are more complex. These structures include the iliotibial band, biceps femoris tendon, patellofemoral ligament, patellar retinaculum, common peroneal nerve, lateral collateral ligament (LCL), fabellofibular ligament, coronary ligament, arcuate ligament, popliteofibular ligament, popliteus tendon, and lateral joint capsule (Figure 1). The primary static stabilizer of the lateral knee is the LCL and rarely constitutes an isolated injury; rather, it generally occurs in conjunction with injuries to the posterolateral corner (PLC). The PLC is comprised of the LCL, arcuate ligament, popliteofibular ligament, fabellofibular ligament, popliteus tendon, and the lateral capsule (6). Injury is commonly seen after motor vehicle accidents as well as in athletics. Injury typically is a result of a valgus force along with knee hyperextension or external tibial rotation (7). The PLC imparts important rotational stability as well.
Physical exam and grading
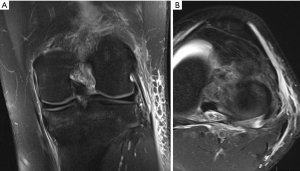
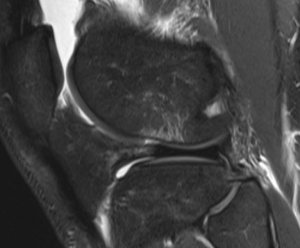
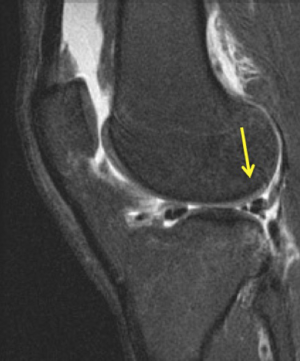
The symptomatology of LCL injury includes swelling, lateral joint line pain, difficulty ascending and descending stairs, and problems with cutting or pivoting. Patients classically ambulate with knee hyperextension or have a varus thrust gait. Lateral joint line tenderness also can occur and patients typically have opening with varus stress at 30 degrees of knee flexion. If there is varus instability at both 0 and 30 degrees, concomitant ACL and/or PCL injury is likely. If patients have increased tibial external rotation at 30 degrees, a combined LCL and PLC injury is probable. A careful neurovascular exam is important, as common peroneal nerve injuries can occur. Grading of the LCL as well as the PLC are quantified by the amount of lateral gapping with varus stress where grade I is defined at 0–5 mm opening, grade II is 5–10 mm, and grade III is >10 mm. Grade I and II indicate a partial tear, while grade III represents a complete tear. Radiographic analysis includes AP, lateral, and varus stress views while MRI is the imaging method of choice to provide information on the location and severity of the soft tissue injury (Figure 2) (7). Although posteromedial in anatomic location, interest in the posteromedial capsular attachment to the medial meniscus has grown, as a tear here, coined a ramp lesion, has been shown to possibly impart important rotational stability as well (Figure 3) (8). With injury to the PLC, meniscocapsular separation medially can occur and also should be investigated. Currently, no physical examination has been found to be sensitive or specific in diagnosing ramp lesions.
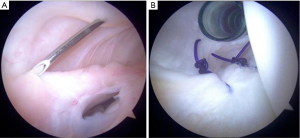
Treatment
Non-operative treatment is often efficacious with grade I or II LCL injuries in isolation. This involves knee bracing followed by physical therapy. Return to sport with these injuries is typically in 6 to 8 weeks after injury (7,9).
Surgery is indicated in grade III injuries, patients with rotatory instability (indicating both an LCL and PLC), and posterolateral instability (indicating LCL/PLC and ACL/PCL injuries). Controversy exists and it is not agreed upon in regard to the best repair and reconstruction sequence of these complex injuries. Improved outcomes result with acute surgery, as long as the soft tissue envelope allows. Successful outcomes have been show if surgery is done within 2 weeks, using suture anchors for avulsion injuries and suture repair alone for those of the midsubstance. In isolated LCL reconstruction, patellar tendon autograft has resulted in good outcomes. In LCL with popliteofibular ligament reconstruction, figure of eight Larson technique is utilized where a hamstring graft is passed through a fibular head bone tunnel which is consequently anchored to the lateral femoral anatomic origin. Another option is the transtibial double-bundle technique. Here, an allograft of the Achilles tendon is fixed to the femoral anatomic origin, and then tendon is split with half then being secured to the fibular head through a bone tunnel, while the other half is attached to the posterior tibia. A thorough physical exam is crucial for prompt diagnosis of complex lesions as this likely improves outcomes (10). Again, knee instability secondary to the improper healing of these injuries are likely to be pain generators and may lead to rotatory instability. The more recent identification of ramp lesions and their possibly role in knee stability has led some to advocate for fixation of these with the possibility that neglect of these lesions may be a cause of failure of ligament reconstruction and continued pain (Figure 3) (8).
ACL injury
Anatomy and biomechanics
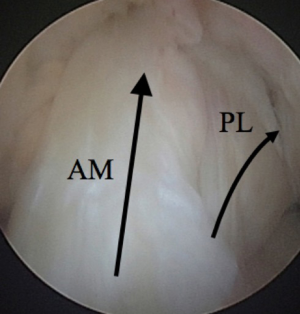
ACL injury may be the greatest contributor to knee instability and pain. The ACL originates at the intercondylar notch on the lateral femoral condyle and its insertion is on the tibial articular surface between the medial and lateral tibial spines. It functions to provide anteroposterior stability, as well as to control rotation of the knee. The ACL is made up of two bundles which are named for their tibial insertion site locations: the anteromedial (AM) and the posterolateral (PL) bundles (Figure 4). The AM bundle is tight in flexion while the PL bundle tightens with knee extension. Acute ACL injuries typically occur as a non-contact pivot type injury, and commonly one feels a “pop” and has immediate swelling and pain. This injury pattern is thought to occur when the knee is in valgus, internal rotation, and 20 degrees of flexion with eccentric quadriceps contraction (11).
Physical exam and grading
On examination, an effusion, which can be painful, is typical and can lead to a quadriceps avoidance gait. The most sensitive exam for ACL injury is the Lachman test. Another commonly used test is the anterior drawer. A KT-1000 device can quantify anteroposterior laxity but not rotational instability, which is also important (11,12). The pivot shift test is best to evaluation rotation and is performed by internally rotating the tibia 20 degrees, then applying a valgus force during which the examiner flexes the knee. A test is considered positive when the tibial plateau reduces and a clunk is sensed at around 20 to 30 degrees of knee flexion (12).
Lateral meniscal tears and MCL injuries are commonly seen with acute ACL injury. Chronic ACL deficiency can also lead to tearing of the menisci from continued knee instability. Chondral injury is also possible. Women more commonly experience ACL injury (4.5:1) for numerous proposed reasons. Baseline valgus alignment, altered landing mechanics (in extension and valgus), quadricep muscle dominance, smaller ligament size, smaller notch size, different hormone levels, and genetic factors related to collagen production have all been implicated as reasons (13).
Initial imaging should involve standard radiographs, which are generally unremarkable. However, one should carefully evaluate for a Segond fracture, which is pathognomonic for an ACL tear. This occurs secondary an anterolateral (AL) capsule avulsion from the proximal lateral tibia (Figure 1). An MRI is recommended for evaluation for an ACL tear, meniscal tears, subchondral damage, loose bodies, and bone bruising (Figure 5), which, according to some studies, can all serve as pain generators. Bone bruising occurs in the lateral compartment in over 80% of ACL ruptures, while the medial compartment is involved in varying percentages (14). The literature is conflicting regarding whether these bone bruises are a source of knee pain. This bruising is thought to occur from the tibia and femur impacting one another during a shifting moment. Typically, this occurs in the middle 1/3 of the lateral femoral condyle as well as the posterior 1/3 of the lateral tibial plateau. In two studies, the only factors shown to be linked to bone bruising are injury during non-jumping activities and younger patients (15,16). The severity of bruising has been thought to be related to the amount of impact and possibly future knee function. Chondrocyte damage has been seen histologically with bone bruising and chondral surface thinning in the area of bruising between 1 to 6 years after injury (17,18). With 12-year follow-up in one of these studies, bone bruise changes resolved and there was no difference in pain (16). One study of 672 patients undergoing ACL reconstruction did not link knee pain or symptoms to a bone bruise at index ACL reconstruction, but did link BMI, older age, female sex, LCL injury, MCL injury, medial meniscus status, lateral meniscus status, laxity on Lachman testing, and chondrosis of the medial, lateral, and anterior compartments statistically. However, of these, only concomitant LCL injury was statistically and clinically significant. Importantly, increased knee pain occurring during ACL reconstruction may lead to more difficult rehabilitation as well as more pain at 2 years post-op according to the multicenter study (15).
Cyclops lesions are also a possible source of pain in the injured knee as well as after ACL reconstruction, when a tuft of tissue on the anterior tibia blocks terminal extension in the intracondylar notch. One study found that 75–86% of patients with this condition had significant pain, and after arthroscopic debridement, these patients regained terminal extension, were pain free at extension, and returned to previous function (19). Some hypothesize that early post-op full knee extension may reduce the incidence of this lesion occurring in ACL reconstructions as the ACL graft may block its formation (20). In the authors’ experience, anatomical and individualized approaches to ACL reconstruction have anecdotally reduced the incidence of cyclops lesions by comparison to conventional methods.
Anterior knee pain is commonly seen with ACL tears, particularly post-operatively. Early weight bearing has led to decreased knee pain compared to patients with delayed weight bearing. It is thought that much of this knee pain is secondary to a flexion contracture as well as quadriceps weakness and therefore regaining early terminal knee extension is critical to preventing anterior knee pain. Early knee extension has been thought to lead to graft loosening by some, but biomechanical studies have shown similar KT-1000 results in patients who underwent more aggressive knee extension. Pre-existing chondromalacia also is a source of anterior knee pain in patients with and without ligamentous knee injuries. This is a common source of anterior knee pain post-operatively after ACL reconstruction as well (20).
Articular cartilage and meniscal injuries are also associated with knee pain in ACL injury. Studies have shown meniscal tears in 41–81% of acute ACL tears and 58–100% in chronically ACL deficient knees, 70% being in the medial meniscus (Figure 6) (21). One study linked a higher BMI to greater intra-articular injury and therefore possibly a poorer outcome while another found females to have fewer intra-articular injuries and therefore may have improved outcomes. In one large study of 780 patients, decreased knee range of motion was linked to increased arthritic changes no matter the status of the meniscus at long term follow-up (22). As mentioned previously, some studies report that bone bruising is not correlated to pain, but rather meniscal, cartilage, and soft tissue injuries are the source of pain with ACL tears.
Treatment
Non-operative treatment of ACL injury includes physical therapy and lifestyle modifications. This, many times, can be appropriate in low demand, older individuals. Recurrent functional instability is still common and therefore a thorough discussion with the patient about realistic outcomes and activity modifications in operative versus non-operative treatment is necessary.
ACL reconstruction is elected in many young and/or active patients. The timing of reconstruction is important, as is appropriate prehabilitation. Preoperative extension and time to surgery has shown to be linked to post-operative terminal extension, pain, and the need for arthroscopic debridement status post ACL reconstruction. Furthermore, having adequate range of motion has led to decreased rates of arthrofibrosis, improved outcomes, and pain (23). The adherence to a specific timing of ACL reconstruction has shifted to a patient specific or individualized determination based on range of motion, swelling, quadriceps control, and pain.
Techniques of ACL reconstruction can also influence pain, whereby donor site pain can be a significant issue. One study found that harvesting >35 mm of tibial tuberosity bone for a bone tendon bone (BTB) technique led to increased pain at 18 months compared to when a 25 mm bone block was harvested (24). Anterior knee pain is quite common with both hamstrings and BTB, while more kneeling pain is seen with BTB. The authors, approach to ACL surgery is based on the principle of anatomy and utilizes an individualized approach to treatment with consideration of each patient’s anatomical characteristics (ACL and graft tissue), as well as lifestyle preferences and concomitant injuries.
Few studies have evaluated the type and number of tunnels and its relationship to pain. One study cites decreased pain with an all inside technique with the thought being less surgical trauma and also more anatomic graft and tunnel placement (25).
Comparing pain with fixation devices, such as interference screw vs. extracortical suspensory fixation, is not well established. Two studies comparing femoral graft fixation with a bioabsorbable interference screw vs. bioabsorbable cross pins showed no difference in pain or outcomes (26,27). There was also no difference in pain at 1 year when comparing bioresorbable interference screw and bioresorbable transfixation devices (28). Another study showed no difference in pain when using EZLoc and the Bone Mulch Screw (Biomet, Warsaw IN) for femoral fixation in hamstring ACL reconstruction (29). In tibial fixation, one study reported that bioabsorbable screw fixation patients had increased pain compared with metal screw fixation with at 2 years. The thought is that complement activation leads to inflammation occurring when the screw integrates (30). The most common issue with pain with interference screws is prominent hardware. One study reported 14 patients at an average of 26 months with pretibial swelling and pain over the bioabsorbable screw (31).
Individual patient differences such as sex, age, height, BMI, chronicity of tear, contact vs. non-contact athletes, and jumping athletes are considerations in regards to knee pain with ACL tears. One study of a Swedish national database noted the mean age for cruciate tears was 32 years and an increased incidence in women less than 20 years old compared with matched male patients. While they noted that a higher number of male cruciate tears, females were more likely to be injured at a younger age (32). The same database was evaluated for outcomes of pain, sport, and quality of life and found that females had significantly worse outcomes compared with males 1 to 2 years after surgery (33). As mentioned, female biomechanics differ and therefore place women at increased risk for knee injury. This is likely multifactorial and may be exacerbated after ACL reconstruction. One study reported that females have a greater knee abduction moment after ACL reconstruction and therefore this places them at risk for further knee injury and pain (34). Another study demonstrated increased anterior-posterior knee shear forces in females status post ACL reconstruction, possibly leading to these same risks and injuries (35).
Body mass index (BMI) has been shown to play a role in ACL tears and therefore knee pain. One large study examined female military cadets and found that BMI of 1 standard deviation or more above the average amplified their risk for ACL tear (36). Height also predisposes athletes to ACL tears, as the center of mass is higher leading to increased difficulty with muscular control and a larger lever arm. This is particularly important in puberty and results in an amplified risk of ACL tear during a growth spurt (37). After puberty, female neuromuscular patterns worsen while that of a male improves, again predisposing females to knee injury (38). Seventy to eighty percent of ACL tears are non-contact injuries; therefore, non-contact sports may predispose a patient to injury. Landing from a jump in or near full extension is a common non-contact ACL injury mechanism.
Failure of ACL reconstruction, whether it is graft failure or persistent instability, can be linked to pain. Reasons for failure and therefore pain include malpositioned tunnels. For example, transtibial drilling with an anterior or “high” femoral tunnel and/or the tibial tunnel being too anterior may lead to decreased extension.
Perioperative therapies, such as continuous passive motion (CPM), ice, bracing, and neuromuscular electrical stimulation have shown varying results in the literature. CPM was thought to possibly improve range of motion and pain; however, the literature shows no difference in pain or outcome and therefore no clinical benefit can be determined, but the CPM does come with a significant financial cost (39). Cryotherapy has been shown to reduce inflammation, induce vasoconstriction, which leads to less swelling, pain and a decrease in hemarthrosis. Its cost effectiveness and low morbidity make cryotherapy an important adjunct in reducing knee pain and improving outcomes (40). As described previously, full knee extension is important in outcomes, as well as pain, and bracing is an effective way to achieve this. Bracing in the post-operative period has been demonstrated to reduce pain, swelling, and the frequency of hemarthrosis as well as wound problems. One proposed goal is to have full extension at the end of surgery and have 90 degrees of flexion by 7 to 10 days. This is obtained by swelling and pain control and early reactivation of the quadriceps, all of which decrease pain and improve outcomes. Electrical muscle stimulation is controversial as an adjunct to exercises to recover muscle strength. This is thought to be secondary to a lack of standardization of protocols of the use of this therapy.
PCL injury
Anatomy and biomechanics
PCL injury occurs in greater frequency after traumatic injury than in sporting activities. Many times, associated injury exists, particularly to the PLC. The PCL is technically extra-articular as the posterior capsular synovium wraps circumferentially around it. It therefore receives blood supply from the synovium in addition to the same vascular supply and innervation as the ACL. The PCL consists of the bigger and stouter AL bundle and the PM bundle. The PM bundle is taut in extension while the AL is taut in flexion. It originates from the anterior cartilage margin of the medial femoral condyle and inserts 1.0 to 1.5 cm distal to the joint on the posterior tibia, juxtaposed to the popliteal artery. Two connect the PCL to the posterior horn of the lateral meniscus. The anterior MFL of Humphrey attaches to the PCL anteriorly, while the posterior MFL of Wrisberg attaches to the PCL posteriorly (41).
Physical exam and grading
Unlike ACL injury, patients typically complain of pain posteriorly and seldom report a “pop” or instability. After resolution of symptoms from the acute injury, patients may have minimal to severe impairment. Patients may complain of pain at all times while others only have discomfort when walking up inclines. Combined ligamentous injuries are characteristically more symptomatic and may lead to feelings of instability.
Classification of PCL injuries are established by chronicity, accompanying injury, as well as the degree of tibial translation. Treatment varies based on if the injury is isolated versus combined making proper diagnosis essential. PCL injuries in isolation often are successfully treated without surgery and have “good to excellent results”, while combined injuries typically have improved outcomes when utilizing early surgical intervention (42). Injury to the PCL is frequently seen in combination with other ligament injury, fractures, as well as with vessel and nerve injuries. Knee dislocation must be considered in combined ACL/PCL injuries or any three-ligament injury. Neurovascular assessment is essential in these cases (43). Chronicity is also critical because PLC scaring will occur if surgery is delayed greater than 3 weeks, making repair difficult. Further, rotatory instability can be problematic due to injury to the capsule (42).
The most precise assessment of PCL injury is the posterior drawer test where, at 90 degrees of flexion, a posteriorly directed force is applied on the proximal tibia. In an uninjured knee, normally a step off anteriorly of 1 cm from the medial femoral condyle to the tibial plateau exists. The grading system mimics that of the ACL where grade I corresponds to translation of 1 to 5 mm while the normal step off remains. A grade II injury has 5 to 10 mm of translation and the condyle and plateau are flush, while grade III exhibits >10 mm of translation with the plateau being posterior to the condyle. This indicates a complete PCL tear. The posterior sag test, also known as Godfrey’s Test, evaluates this step off which is created by the tibial weight shifting the plateau posteriorly. The quadriceps activation test also is useful for evaluating PCL injury and is done with the knee at 60 degrees. During quadriceps activation, if a grade III tear exists, the tibia reduces anteriorly. The dial test is also essential to assess concomitant PLC injury, as the treatment and outcome will be different. Beyond concomitant ligamentous injury, evaluation of the menisci is essential. Careful and complete MRI review is important (Figure 7).
Treatment
Controversy exists regarding non-operative and surgical treatment of isolated PCL injuries. This is likely due to PCL injuries being less common, meaning fewer large long-term follow-up reports are published. The literature has shown that acute partial PCL tears in isolation have suitable results after non-operative treatment (42,44). However, surgery may be of benefit if painful knee instability exists as meniscal and chondral damage may occur. The PCL retains healing potential, leading to some recommending extension bracing in an attempt to allow the PCL to heal. Treatments are better defined in the less commonly seen PCL avulsion fracture. Minimally displaced fractures are typically successfully treated with immobilization, while displaced fractures require surgery. One kinematic study has shown, however, that with isolated PCL injuries, during stair ascent, the tibia subluxes posteriorly and then reduces meaning shear forces may lead to further damage in a PCL deficient knee (45). This could suggest that more attention be paid to PCL deficient knees, as continued knee instability may lead to further structural damage to the knee, as well as pain.
Multiple graft and fixation options exist for the treatment of PCL injuries, without any clearly superior choice. Both arthroscopic transtibial and open tibial inlay techniques have their own strengths, weaknesses, and risks. Controversy also exists with single- vs. double-bundle reconstruction. Most commonly, an isolated AL single-bundle is reconstructed.
A high tibial osteotomy should be considered in chronic PCL deficiency as unloading the medial compartment and increasing the tibial slope will make the knee more stable. This helps reduce varus angulation and posterior tibial sag. Individualized treatment is essential in both isolated PCL and multi-ligamentous injuries as a widespread assortment of injuries exist, which may lead to different results.
Multi-ligamentous knee injury
Anatomy and biomechanics
Multi-ligament knee injuries may occur due to either low or high energy mechanisms and commonly lead to the orthopaedic emergency of knee dislocation. A dislocation usually involves tearing of both the ACL and PCL, along with either or potentially both the MCL and LCL. As one would expect, meniscal and capsular injury are common. Tibial plateau and distal femur fractures are also common, as is damage to the neurovascular structures.
Both osseous and ligamentous anatomy play a major role in overall knee stability. As outlined previously, the major ligamentous stabilizers include the ACL, PCL, collateral ligaments, and PM as well as the PLC. The popliteal fossa contains the popliteal artery and vein, separated only by a thin fatty layer to the posterior joint capsule. More superficial lays the tibial nerve. As the sciatic nerve enters the popliteal space, it becomes the tibial nerves and common peroneal nerve, the later that courses laterally around the fibular head (46). The popliteal artery is tethered to the adductor hiatus proximally and by the soleus distally, making the artery susceptible to injury with knee dislocations.
Physical exam and grading
As vascular injury is relatively common with multi-ligamentous knee injuries and knee dislocations, a careful neurovascular exam is required and of paramount importance. The opposite leg should be used as a control in palpating pulses. Doppler examination and ankle brachial indexes (ABIs) should be performed if a dislocation or subluxation is suspected. One also cannot rule out vascular injury if pulses initially are normal, as intimal tears may have a delayed presentation. This makes serial neurovascular exams and ABIs important. If there is question of vascular status, urgent vascular studies should be undertaken.
Nerves about the knee are less tethered in comparison with the vascular structures, making injury to them less commonly injured but still important to properly evaluate. Nerve injuries are still quite common with knee dislocations, particularly with the peroneal nerve as it courses over the bony fibular head. This nerve is commonly injured in combination with the LCL and PLC. Neuropraxia is more common from stretching of the nerve by comparison to a laceration or rupture (47). However, nerve recovery is unpredictable. Neurologically, it is important to differentiate between stocking paresthesias and a specific distribution, as compartment syndrome is a consideration with these injuries. Delayed presentation also occurs with swelling or hematoma formation (46,47). In this regard, electromyography (EMG) and nerve conduction studies may shed light onto these nerve injuries a few weeks after injury (46). As one would suspect, injury to these structures, nerve regeneration, and the possibility of chronic regional pain syndrome can be devastating and are all possible causes of pain. Further, iatrogenic injury to neurovascular structures can also occur from immobilization and surgical intervention and can cause poor outcomes including chronic pain.
The most commonly used classification of knee dislocations is established based on the direction of displacement of the proximal tibia in relation to the femur. Many times, dislocations may be missed if they reduce on their own before presentation. Mechanism, neurovascular exam, and degree of displacement may be clues for a reduced knee dislocation. Special attention should be paid to the skin for lacerations, dimpling, and color changes that may necessitate more urgent operative intervention. As these injuries often occur as a result from high energy injury, comprehensive primary and secondary exams are important (48).
Intuitively, knee pain following a multi-ligamentous knee injury is common. With intraarticular tibial plateau and distal femur fractures, early post-traumatic osteoarthritis is anticipated due to abnormal wear patterns. Articular cartilage and meniscal injury is common, resulting in an increased possibility of loose bodies and other pain generators (Figure 8). Scar formation with multiple injuries commonly leads to arthrofibrosis. If the ligamentous structures do not heal properly, the knee can also continue to be unstable and possibly lead to pain and poor outcomes. As more structures are injured, complications and pain are more likely and therefore create a more complex clinical scenario.
Treatment
Nerve and vascular injury are the most urgent considerations if they exist. A vascular surgery consultation is important if vascular injury occurs for possible repair and/or bypass. It is recommended that nerve palsies be initially observed. Electrodiagnostic testing should be performed at 6 weeks and 3 months if re-innervation is an issue. If at 3 months no recovery is seen, exploration with possible neurolysis can be considered. If partial or complete rupture of the nerve exists, referral to a microsurgeon should be done within 3 months. If complete transection of a nerve exists and primary repair can be done without tension acutely, it can be undertaken with the ligamentous structures being dealt with after about 3 weeks.
Specific treatment recommendations have not been well established and are surgeon specific. One meta-analysis found that in 132 knee dislocations, surgical intervention was related to better outcomes when compared with non-operative treatments, although morbidity is still quite common (49). Few patients are treated non-operatively unless patients are unable to tolerate a surgical procedure or are very low demand. Non-operative options include a long-leg, cylinder cast, or a long leg brace locked in extension. If non-operative treatment is elected, knee reduction must be confirmed radiographically.
Operative intervention and timing are controversial and surgeon dependent, as literature varies and few large studies exist. Knee-spanning external fixators can provide more stability than bracing and therefore represent a good option, especially in poor surgical candidates. Irrigation and debridement of wounds as well as vascular and neurologic interventions should take priority. Fractures should also be treated before any ligamentous surgery is performed. Some believe that delaying any ligamentous surgery for 1 to 3 weeks to observe vascular and neurologic status is preferred. Many times, PCL repair or reconstruction is usually undertaken first with delayed treatment of ACL and possible collateral ligaments if necessary in an attempt to reduce arthrofibrosis and therefore improve outcomes and decrease pain. Some advocate for acute intervention, citing better postoperative results. Many combinations of operative and non-operative treatments exist for multi-ligamentous knee injuries without a clearly superior algorithm with common outcomes that include continued knee instability and pain (50).
Conclusions
Knee pain plays a noteworthy part in the outcome of the unstable knee, much of which is related to ligamentous injury and the consequential injury to other knee structures. ACL tears lead to knee pain that can be secondary to hemarthrosis, articular injuries, meniscal pathology and/or patellofemoral pain. Controversy exits regarding the role bone bruising plays in ACL injured patients. Patient differences in knee pain such as sex, age, BMI, and type of sport play a role as well but must be further defined. When ACL reconstruction is chosen, prehabilitation is vital in decreasing the risk of arthrofibrosis and post-operative knee pain. Donor site pain, as well as painful hardware, also is relevant. Post-operative therapies are also critical in preventing and dealing with knee pain. PCL injury also leads to pain and can lead to instability, even asymptomatically. As with the ACL and via the same mechanisms, this could lead to further cartilage damage and osteoarthritis leading to knee pain. Multi-ligamentous knee injuries are complex and special attention must be paid to the vascular and neurologic status of the patient. Complications, arthrofibrosis, and pain are common issues with these injuries and no superior treatment scheme has been well established. The authors recommend an individualized approach with a thorough exam, appropriately indicated treatments, and post-operative care to prevent further injury and debilitating pain to allow the best outcomes.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: FFH serves as an Editor-in-Chief of Annals of Joint from Mar 2016 to Aug 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Halinen J, Lindahl J, Hirvensalo E, et al. Operative and nonoperative treatments of medial collateral ligament rupture with early anterior cruciate ligament reconstruction: a prospective randomized study. Am J Sports Med 2006;34:1134-40. [Crossref] [PubMed]
- LaPrade RF, Engebretsen AH, Ly TV, et al. The anatomy of the medial part of the knee. J Bone Joint Surg Am 2007;89:2000-10. [PubMed]
- Singhal M PJ, Johnson D. Medial ligament injuries. In: Delee JC, Drez D, Miller MD, editors. Delee and Drez's orthopaedic sports medicine, principles and practice. 3rd ed. Philadelphia: Saunders Elsevier; 2010.
- Kannus P. Long-term results of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res 1988;103-12. [PubMed]
- Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Brace wear preferences and injury risk. Am J Sports Med 1994;22:2-11. [Crossref] [PubMed]
- LaPrade RF, Ly TV, Wentorf FA, et al. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med 2003;31:854-60. [Crossref] [PubMed]
- Cooper JM, McAndrews PT, LaPrade RF. Posterolateral corner injuries of the knee: anatomy, diagnosis, and treatment. Sports Med Arthrosc 2006;14:213-20. [Crossref] [PubMed]
- Chahla J, Dean CS, Moatshe G, et al. Meniscal Ramp Lesions: Anatomy, Incidence, Diagnosis, and Treatment. Orthop J Sports Med 2016;4:2325967116657815 [Crossref] [PubMed]
- Kannus P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med 1989;17:83-8. [Crossref] [PubMed]
- Schorfhaar AJ, Mair JJ, Fetzer GB, et al. Lateral and posterolateral injuries oft he knee. In: DeLee JC, Drez D, Miller MD, editors. DeLee and Drez's Orthopaedic sports medicine. 3rd ed. Philadelphia: Elsevier Saunders; 2009;1718-47.
- Chhabra A, Starman JS, Ferretti M, et al. Anatomic, radiograpshic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am 2006;88:2-10. [PubMed]
- Hoshino Y, Araujo P, Ahlden M, et al. Quantitative evaluation of the pivot shift by image analysis using the iPad. Knee Surg Sports Traumatol Arthrosc 2013;21:975-80. [Crossref] [PubMed]
- Huston LJ, Greenfield ML, Wojtys EM. Anterior cruciate ligament injuries in the female athlete. Potential risk factors. Clin Orthop Relat Res 2000;50-63. [Crossref] [PubMed]
- Speer KP, Spritzer CE, Bassett FH 3rd, et al. Osseous injury associated with acute tears of the anterior cruciate ligament. Am J Sports Med 1992;20:382-9. [Crossref] [PubMed]
- Dunn WR, Spindler KP, Amendola A, et al. Which preoperative factors, including bone bruise, are associated with knee pain/symptoms at index anterior cruciate ligament reconstruction (ACLR)? A Multicenter Orthopaedic Outcomes Network (MOON) ACLR Cohort Study. Am J Sports Med 2010;38:1778-87. [Crossref] [PubMed]
- Hanypsiak BT, Spindler KP, Rothrock CR, et al. Twelve-year follow-up on anterior cruciate ligament reconstruction: long-term outcomes of prospectively studied osseous and articular injuries. Am J Sports Med 2008;36:671-7. [Crossref] [PubMed]
- Viskontas DG, Giuffre BM, Duggal N, et al. Bone bruises associated with ACL rupture: correlation with injury mechanism. Am J Sports Med 2008;36:927-33. [Crossref] [PubMed]
- Johnson DL, Urban WP Jr, Caborn DN, et al. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med 1998;26:409-14. [Crossref] [PubMed]
- Tonin M, Saciri V, Veselko M, et al. Progressive loss of knee extension after injury. Cyclops syndrome due to a lesion of the anterior cruciate ligament. Am J Sports Med 2001;29:545-9. [Crossref] [PubMed]
- Shelbourne KD, Trumper RV. Preventing anterior knee pain after anterior cruciate ligament reconstruction. Am J Sports Med 1997;25:41-7. [Crossref] [PubMed]
- Honkamp NJ, Shen W, Okeke N, et al. Anterior cruciate ligament injuries: 1. Anterior cruciate ligament injuries in the adult. In: DeLee JC, Drez D Jr, Miller MD, editors. DeLee and Drez’s Orthopaedic Sports Medicine. 3rd ed. Philadelphia: Saunders Elsevier; 2009.
- Shelbourne KD, Urch SE, Gray T, et al. Loss of normal knee motion after anterior cruciate ligament reconstruction is associated with radiographic arthritic changes after surgery. Am J Sports Med 2012;40:108-13. [Crossref] [PubMed]
- Mauro CS, Irrgang JJ, Williams BA, et al. Loss of extension following anterior cruciate ligament reconstruction: analysis of incidence and etiology using IKDC criteria. Arthroscopy 2008;24:146-53. [Crossref] [PubMed]
- Aglietti P, Zaccherotti G, Simeone AJ, et al. Anatomic versus non-anatomic tibial fixation in anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Knee Surg Sports Traumatol Arthrosc 1998;6:S43-8. [Crossref] [PubMed]
- Benea H, d'Astorg H, Klouche S, et al. Pain evaluation after all-inside anterior cruciate ligament reconstruction and short term functional results of a prospective randomized study. Knee 2014;21:102-6. [Crossref] [PubMed]
- Frosch S, Rittstieg A, Balcarek P, et al. Bioabsorbable interference screw versus bioabsorbable cross pins: influence of femoral graft fixation on the clinical outcome after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 2012;20:2251-6. [Crossref] [PubMed]
- Capuano L, Hardy P, Longo UG, et al. No difference in clinical results between femoral transfixation and bio-interference screw fixation in hamstring tendon ACL reconstruction. A preliminary study. Knee 2008;15:174-9. [Crossref] [PubMed]
- Rose T, Hepp P, Venus J, et al. Prospective randomized clinical comparison of femoral transfixation versus bioscrew fixation in hamstring tendon ACL reconstruction--a preliminary report. Knee Surg Sports Traumatol Arthrosc 2006;14:730-8. [Crossref] [PubMed]
- Gifstad T, Drogset JO, Grontvedt T, et al. Femoral fixation of hamstring tendon grafts in ACL reconstructions: the 2-year follow-up results of a prospective randomized controlled study. Knee Surg Sports Traumatol Arthrosc 2014;22:2153-62. [Crossref] [PubMed]
- Benedetto KP, Fellinger M, Lim TE, et al. A new bioabsorbable interference screw: preliminary results of a prospective, multicenter, randomized clinical trial. Arthroscopy 2000;16:41-8. [Crossref] [PubMed]
- Ramsingh V, Prasad N, Lewis M. Pre-tibial reaction to biointerference screw in anterior cruciate ligament reconstruction. Knee 2014;21:91-4. [Crossref] [PubMed]
- Nordenvall R, Bahmanyar S, Adami J, et al. A population-based nationwide study of cruciate ligament injury in Sweden, 2001-2009: incidence, treatment, and sex differences. Am J Sports Med 2012;40:1808-13. [Crossref] [PubMed]
- Ageberg E, Forssblad M, Herbertsson P, et al. Sex differences in patient-reported outcomes after anterior cruciate ligament reconstruction: data from the Swedish knee ligament register. Am J Sports Med 2010;38:1334-42. [Crossref] [PubMed]
- Ortiz A, Olson S, Trudelle-Jackson E, et al. Landing mechanics during side hopping and crossover hopping maneuvers in noninjured women and women with anterior cruciate ligament reconstruction. PM R 2011;3:13-20. [Crossref] [PubMed]
- Ortiz A, Olson S, Libby CL, et al. Landing mechanics between noninjured women and women with anterior cruciate ligament reconstruction during 2 jump tasks. Am J Sports Med 2008;36:149-57. [Crossref] [PubMed]
- Uhorchak JM, Scoville CR, Williams GN, et al. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med 2003;31:831-42. [Crossref] [PubMed]
- Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am 2004;86-a:1601-8.
- Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med 2005;33:492-501. [Crossref] [PubMed]
- Wright RW, Preston E, Fleming BC, et al. A systematic review of anterior cruciate ligament reconstruction rehabilitation: part I: continuous passive motion, early weight bearing, postoperative bracing, and home-based rehabilitation. J Knee Surg 2008;21:217-24. [Crossref] [PubMed]
- Raynor MC, Pietrobon R, Guller U, et al. Cryotherapy after ACL reconstruction: a meta-analysis. J Knee Surg 2005;18:123-9. [Crossref] [PubMed]
- Amis AA, Gupte CM, Bull AM, et al. Anatomy of the posterior cruciate ligament and the meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc 2006;14:257-63. [Crossref] [PubMed]
- Shino K, Horibe S, Nakata K, et al. Conservative treatment of isolated injuries to the posterior cruciate ligament in athletes. J Bone Joint Surg Br 1995;77:895-900. [PubMed]
- Schulz MS, Russe K, Weiler A, et al. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg 2003;123:186-91. [Crossref] [PubMed]
- Boynton MD, Tietjens BR. Long-term followup of the untreated isolated posterior cruciate ligament-deficient knee. Am J Sports Med 1996;24:306-10. [Crossref] [PubMed]
- Goyal K, Tashman S, Wang JH, et al. In vivo analysis of the isolated posterior cruciate ligament-deficient knee during functional activities. Am J Sports Med 2012;40:777-85. [Crossref] [PubMed]
- Fanelli GC, Harris JD, Tomaszewski DJ, et al. Reinheimer KN: Multiple ligament knee injuries. In: DeLee. JC, Drez D Jr, Miller MD, editors. DeLee & Drez's Orthopaedic. Sports Medicine: Principles and Practice. 3rd ed. Philadelphia: Saunders, 2009;1747-65.
- Medina O, Arom GA, Yeranosian MG, et al. Vascular and Nerve Injury After Knee Dislocation: A Systematic Review. Clin Orthop Relat Res 2014;472:2621-9. [Crossref] [PubMed]
- Merritt AL, Wahl C. Initial assessment of the acute and chronic multiple-ligament injured (dislocated) knee. Sports Med Arthrosc 2011;19:93-103. [Crossref] [PubMed]
- Dedmond BT, Almekinders LC. Operative versus nonoperative treatment of knee dislocations: a meta-analysis. Am J Knee Surg 2001;14:33-8. [PubMed]
- Peskun CJ, Whelan DB. Outcomes of operative and nonoperative treatment of multiligament knee injuries: an evidence-based review. Sports Med Arthrosc 2011;19:167-73. [Crossref] [PubMed]
Cite this article as: Arner JW, Jiang KN, Musahl V, Fu FH. Pain and the unstable knee. Ann Joint 2017;2:82.