
Allograft augmentation of hamstring autografts was not a cost-effective treatment option for middle aged patients undergoing primary anterior cruciate ligament reconstruction
Introduction
By lessening the rate of graft failure, augmentation of hamstring autografts (HAM) with semitendinosus allograft tissue has been demonstrated to provide a cost-effective treatment option for adolescent anterior cruciate ligament (ACL) reconstruction patients (1). However, with the overall rate of graft failure being much lower in older patients (2), it remains unclear if these so-called hybrid hamstring grafts are a cost-effective option in the middle-aged patient population. As such, the purpose of this study was to compare clinical results and cost-effectiveness between HAM and so-called hybrid HAM augmented with semitendinosus allograft tissue in patients aged 25 years or greater in order to further establish evidence-based patient selection criteria for allograft augmentation. We hypothesized that hybrid grafts would not result in a clinically meaningful reduction in the prevalence of graft failure and, as such, would not be a cost-effective treatment option for middle-aged patients.
Methods
Patients
This study received IRB approval from our institution (protocol # 16-0077-P1H) and the current analysis included all patients aged 25 years or older that had undergone primary ACL reconstruction between 2010 and 2015 with HAM, either with or without allograft semitendinosus augmentation. Patients undergoing multi-ligament reconstruction or where revision anterior cruciate ligament reconstruction (ACLR) was the index procedure at our institution were excluded. Patients were not excluded based on the condition or treatment of the menisci or articular cartilage.
To compare clinical results of the two grafts, we identified patients ≥25 years old that had undergone ACLR by a single surgeon between 2010 and 2015 with either a HAM or hybrid autograft with semitendinosus allograft augment (HYBRID). Similar surgical technique, pre- and postoperative rehabilitation were used for both groups as directed by the single senior surgeon.
Surgical technique
As previously described, the gracilis and semitendinosus autograft harvest was identical between those receiving either HAM or HYBRID grafts (1). The gracilis and semitendinosus tendons were harvested using standard techniques, and were then either quadrupled or quintupled (3). For those having a HYBRID graft, the allograft tissue was whip-stitched on both ends. The allograft tissue was sterilized using the Allowash XG process including 1.2 to 1.9 Mrad irradiation (LifeNet Health, Inc., Virginia Beach, VA) (4). The allograft tissue was passed first with the autograft tissue then being placed around the allograft with the end goal of having as little of the allograft tissue exposed to the knee joint milieu in vivo. With the allograft strands placed in the middle of the autograft strands, the entire graft was doubled over forming six strands (1).
For both HAM and HYBRID grafts, the femoral tunnel was drilled using an accessory anteromedial portal. Femoral fixation was achieved using a suspensory fixation (Endobutton, Smith & Nephew, Andover, MA) and a BIORCI (Smith & Nephew, Andover, MA) bioabsorbable interference screw was used for tibial fixation with the free tendon ends wrapped around a bi-cortical 4.5 mm large fragment screw with a 20 mm washer (Geofit, Depuy Mitek, Norwood, MA) for backup fixation.
Statistical analyses
We retrospectively collected patient demographic information and the need for subsequent surgeries to the involved knee. In addition to the need for revision ACLR, we also included evidence of a failed graft on magnetic resonance imaging, or pathological laxity during the physical examination (positive Lachman test, marked anterior laxity, or a positive pivot shift test compared to the healthy knee), or patient-reported instability that affected the patient’s ability to perform activities of daily living or sporting activities, in our definition of a failed graft. Any patient that met these criteria was considered to have a failed graft regardless of whether the patient chose to undergo revision ACL reconstruction (1,5,6). Continuous variables such as age and BMI were compared with independent t-tests and categorical variables such as sex and graft failure were compared between HAM and HYBRID groups using two-tailed χ2 or Fisher Exact tests as appropriate. For all analyses, an α-level of P≤0.05 was considered statistically significant.
The clinical results were then used to compare the cost-effectiveness between HAM and HYBRID grafts when used in series of middle-aged patients. We utilized previously published methods and this study made the following assumptions: (I) patients underwent equivalent preoperative treatment with equivalent costs; (II) patients did not suffer contralateral ACL injury during the study period; and (III) those with complications or graft failures occurring after a tertiary surgery remained at the current health state (primary surgery = index ACL reconstruction, secondary surgery = revision ACL reconstruction or reoperation due to infection or arthrofibrosis, tertiary surgery = second revision ACL reconstruction or second reoperation for infection or arthrofibrosis; Figure 1).
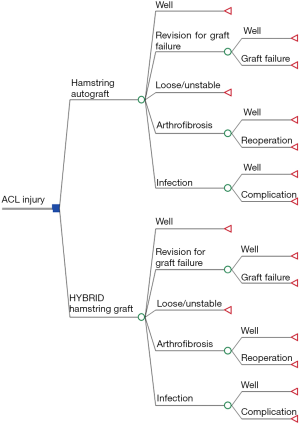
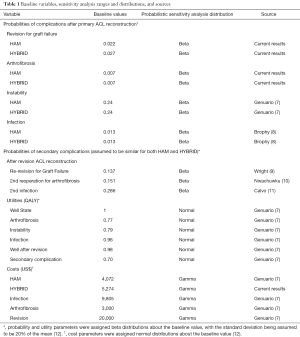
The base case cost-effectiveness was assessed using Monte Carlo microsimulation of a simplified decision tree model created with 1,000 theoretical patients assigned equally to HAM or HYBRID cohorts (Figure 1). These treatment arms were divided into outcome arms based on probabilities, utilities, and costs derived from both the current clinical results and from the literature (Table 1). Specifically, we included the probabilities of a successful outcome as well as revision due to graft failure, persistent instability, arthrofibrosis requiring reoperation, and infection. These values were taken directly from our clinical results or previously published literature on adult patients (Table 1). The incremental cost of the HYBRID graft was $1201.56 greater than the HAM graft based on pricing at our facility. All other costs were consistent with the previous study by Genuario et al. (7). Terminal outcomes were assigned a health state/utility score and a societal cost, and the threshold for cost-effectiveness was set as an incremental cost-effectiveness ratio of $50,000/quality-adjusted life year (QALY) (7).
Full table
To better estimate uncertainty within each model, probabilistic sensitivity analysis of 1,000 first-order cases was used to vary probabilities, utilities and costs within the two models. Similar to the methods of Crall et al. (12) and Jacobs et al. (1), probability parameters were assigned beta distributions, utility parameters were assigned normal distributions, and cost parameters were assigned gamma distributions. All parameter distributions were centered about the baseline value and standard deviations were assumed to be 20% of the mean. We also determined the percentage of cases in which each strategy (HAM or HYBRID graft) would be the preferred strategy based on the willingness to pay (WTP) of $50,000.
Results
Clinical results
There were a total of 288 patients, 138 patients in the HAM group and 150 in the HYBRID group (Table 2). The two groups did not differ in terms of age; however, the groups did differ in sex, BMI, and the duration of follow-up with the HAM group having significantly fewer females, lower BMI, and longer follow-up (Table 2). The prevalence of graft failure did not differ between groups (HAM = 3/138, 2.2% vs. HYBRID = 3/150, 2.7%, P>0.99).

Full table
Cost-effectiveness results
The HAM graft was the dominant strategy in the base case as it provided an incremental cost savings of $1,329.92 with both treatment options demonstrating equal effectiveness (QALY = 0.94 for both groups). Probabilistic sensitivity analysis results were similar, with the HAM graft demonstrating mean costs of $4,736.86±$860.48 compared to $6,059.25±$1,056.04 for the HYBRID group. Again, both groups demonstrated similar effectiveness (QALY =0.94±0.15). The combination of lower cost and similar effectiveness made the HAM graft the preferred treatment strategy in 85% of the simulated cases.
Discussion
The purpose of this study was to compare clinical results and cost-effectiveness between HAM and hybrid hamstring autografts augmented with semitendinosus allograft tissue in patients aged 25 years or greater in order to further establish evidence-based patient selection criteria for allograft augmentation. Our hypothesis that hybrid grafts would not result in a clinically meaningful reduction in the prevalence of graft failure and, as such, would not be a cost-effective treatment option for middle-aged patients was supported by the current results.
There are limited published reports that have assessed the efficacy of hybrid HAM, and the current results represent the largest study in terms of the number of patients treated with hybrid grafts to date. In addition to the current results there have been four publications on the topic—two in adolescent patient populations (1,13) and two in more general populations (4,14). We have previously reported significantly reduced failure rates with hybrid grafts in select subsets of adolescent patients (1) and the current results suggest equitable efficacy between HAM and hybrid grafts in middle-aged patients. On the contrary, we recognize that all published results outside of our institution have reported inferior results with hybrid grafts. While there were differences between studies in terms of allograft tissue source (semitendinosus vs. Achilles tendon) and sterilization methods, we suggest that the dramatically different clinical results may have been due to differences in graft preparation technique. Specifically, the location of the allograft tissue within the graft may be important. Our technique involves incorporating the allograft tissue within the autograft tissue, theoretically minimizing the amount of allograft tissue exposed to the knee joint milieu. Unfortunately, the specific location of the allograft tissue within the hybrid graft was not clearly described by Darnley et al. or Pennock et al. (13,14); however, Burrus et al. (4), who reported significantly greater graft failure with hybrid grafts, stated that the hybrid grafts were rotated to place the allograft tissue anteriorly. Both on second-look arthroscopy and MRI, they reported that it was, indeed the anterior fibers of their hybrid grafts that had failed (4). Furthermore, Pennock et al. reported that hybrid grafts tended to fail during the first postoperative year, and suggested that hybrid grafts may take longer to incorporate (13). The marked differences in clinical performance between our institution and others, combined with mean time to failure of more than 18 months in both our adolescent and middle-aged series, suggest that graft preparation methods that protect the allograft tissue may improve incorporation of the hybrid graft, thereby improving clinical performance.
The current results represent an important step in defining appropriate patient selection criteria for hybrid grafts. We have previously reported that hybrid grafts significantly reduced graft failure rates in skeletally immature patients as well as those patients under the age 18 that the surgeon felt might have greater difficulty with postoperative rehabilitation following bone-patellar tendon-bone graft harvest (1). Some have expressed concern that augmenting a HAM in a small female patient may result in graft impingement; however, this was not borne out as there was no difference in reoperations for cyclops lesions, arthrofibrosis, or limited knee extension range of motion between the two grafts (1). The hybrid graft successfully reduced graft failure rates for both adolescent males (HYBRID: 4/24, 17% vs. HAM: 6/20, 30%) and females alike (HYBRID: 1/18, 6% vs. HAM: 7/26, 27%) (1), further suggesting that theoretical risk of graft impingement was not realized clinically. While this was the case, we do caution that the size of the graft must be customized in the small adolescent female or skeletally immature patient. We are in no way suggesting a one-size-fits-all approach be taken, or that all graft diameters should be ≥10 mm. On the contrary, the patient’s anatomy and sporting demands should be taken into account so that the same graft would likely not be used in an 80 lb 16-year-old skeletally mature female vs. 200 lb 13-year-old skeletally-immature male.
While previously demonstrated to significantly reduce graft failure rates in subsets of adolescent patients, the current results suggest that routine allograft augmentation may not be necessary in patients aged 25 years or older. Hybrid grafts did not yield inferior results in the middle-aged patients, but rather that the increased initial cost of the allograft tissue was not offset by a reduction in failure rates as was seen in adolescent patients. It is important to note that the current results suggest that routine allograft of all middle-aged patients is not cost-effective but that there may be subsets of middle-aged patients for whom hybrid grafts are indicated. While HAM were the dominant treatment strategy, the probabilistic sensitivity analysis results indicated that hybrid grafts were the preferred strategy in 15% of simulated cases. This suggests that there may very well be subsets of middle-aged patients that may be better served with a hybrid graft. Future studies are then necessary to determine if patients that place greater stress on the graft postoperatively (i.e., patients that resume cutting sports and/or those with greater BMI, etc.) may benefit from a hybrid graft.
This study was not without limitation. This study, while the largest sample of hybrid grafts reported in the literature to date, did not include patient-reported outcomes. Larger prospective studies are necessary to validate the current findings and examine potential effects on patient-reported outcomes. We also did not routinely capture postoperative sports participation and activity levels, which limited our ability to identify a potential subset of middle-aged patients that may benefit from hybrid graft selection. Second, this analysis was based on the United States healthcare system. Allograft augmentation may not be an available treatment option in other geographic locations, and the cost-effectiveness results may differ in other healthcare environments.
In conclusion, the cost-effectiveness of hybrid grafts previously seen in adolescent patients was not realized in an older patient population. Routinely augmenting HAM with semitendinosus allograft tissue did not result in a reduced rate of graft failure and, as such, was not a cost-effective treatment option for middle-aged patients when compared to HAM. HAM provided incremental cost savings of $1,322.39 per patient compared to the hybrid graft, and the HAM grafts were the preferred strategy in 85% of simulated cases. This information can assist surgeons as both the techniques and patient selection criteria for hybrid grafts continue to evolve.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Freddie H. Fu and Jeremy M. Burnham) for the series “Trends in ACL Reconstruction” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2017.05.05). The series “Trends in ACL Reconstruction” was commissioned by the editorial office without any funding or sponsorship. DLJ reports grants and personal fees from Smith & Nephew, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study received IRB approval from our institution (protocol # 16-0077-P1H).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jacobs CA, Burnham JM, Makhni EC, et al. Allograft augmentation of hamstring autograft for younger patients undergoing anterior cruciate ligament reconstruction: clinical and cost-effective analyses. Am J Sports Med 2016;ePub ahead of print.
- Magnussen RA, Lawrence TR, West RL, et al. Graft size and patient age are predictors of early revisions after anterior cruciate ligament reconstruction with hamstring autograft. Arthroscopy 2012;28:526-31. [Crossref] [PubMed]
- Hamner DL, Brown CH Jr, Steiner ME, et al. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning results. J Bone Joint Surg (Am) 1999;81:549-57. [Crossref] [PubMed]
- Burrus MT, Werner BC, Crow AJ, et al. Increased failure rates after anterior cruciate ligament reconstruction with soft-tissue autograft-allograft hybrid grafts. Arthroscopy 2015;31:2342-51. [Crossref] [PubMed]
- van Eck CF, Schkrohowsky JG, Working ZM, et al. Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med 2012;40:800-7. [Crossref] [PubMed]
- Noyes FR, Barber-Westin SD. Revision anterior cruciate surgery with use of bone-patellar tendon-bone autogenous grafts. J Bone Joint Surg (Am) 2001;83-A:1131-43. [Crossref] [PubMed]
- Genuario JW, Faucett SC, Boublik M, et al. A cost-effectiveness analysis comparing 3 anterior cruciate ligament graft types: bone-patellar tendon-bone autograft, hamstring autograft, and allograft. Am J Sports Med 2012;40:307-14. [Crossref] [PubMed]
- Brophy RH, Wright RW, Huston LJ, et al. Factors associated with infection following anterior cruciate ligament reconstruction. J Bone Joint Surg (Am) 2015;97:450-4. [Crossref] [PubMed]
- Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg (Am) 2012;94:531-6. [Crossref] [PubMed]
- Nwachukwu BU, McFeely ED, Nasreddine A, et al. Arthrofibrosis after anterior cruciate ligament reconstruction in children and adolescents. J Pediatr Orthop 2011;31:811-7. [Crossref] [PubMed]
- Calvo R, Figueroa D, Anastasiadis Z, et al. Septic arthritis in ACL reconstruction surgery with hamstrings autografts. Eleven years of experience. Knee 2014;21:717-20. [Crossref] [PubMed]
- Crall TS, Bishop JA, Guttman D, et al. Cost-effectiveness analysis of primary arthrscopic stabilization versus nonoperative treatment for first-time anterior glenohumeral dislocations. Arthroscopy 2012;28:1755-65. [Crossref] [PubMed]
- Pennock AT, Ho B, Parvanta K, et al. Does allograft augmentation of small-diameter hamstring autograft ACL grafts reduce the incidence of graft retrear? Am J Sports Med 2017;45:334-8. [Crossref] [PubMed]
- Darnley JE, Leger-St-Jean B, Pedroza AD, et al. Anterior cruciate ligament reconstruction using a combination of autograft and allograft tendon: a MOON cohort study. Orthop J Sports Med 2016;4:2325967116662249 [Crossref] [PubMed]
Cite this article as: Jacobs CA, Malempati CS, Makhni EC, Johnson DL. Allograft augmentation of hamstring autografts was not a cost-effective treatment option for middle aged patients undergoing primary anterior cruciate ligament reconstruction. Ann Joint 2017;2:24.


