
Strategies in managing the labrum
Anatomy
Hip joint anatomy (form) and biomechanics (function) are significantly impacted by the fibrocartilaginous acetabular labrum. Largely oversimplified as a “ball-and-socket”, the hip is a highly complex structure better described as a layered concept of four layers (1). In layer II (inert), the labrum, which increases the surface area (22%) and volume (33%) of acetabular coverage, provides a suction seal to the femoroacetabular articulation (2). The labrum increases socket depth and joint congruity, which leads to increased stability. Thus, the primary aim of labral surgery should be to anatomically restore its form and preserve its function.
The labral suction seal has been the source of much investigation. It is this fluid seal that permits production of a negative intra-articular pressure, enhancing joint stability (3). Synovial fluid transport out of the central compartment is directly controlled by the labrum in vivo (4). The seal, between the labrum and femoral head, is significantly affected by position (i.e., hip range of motion). Greater degrees of flexion and internal rotation and pivoting have been shown to increase fluid transport from the central to the peripheral compartment, negating the suction seal, and decreasing femoral head stability (4-6). Similarly, greater degrees of abduction change the shape of the labrum (length and cross-sectional area) and significantly increase its resultant strain (7). As femoroacetabular impingement (FAI) syndrome is a motion- and position-dependent entity, the “anterior impingement” position (flexion, adduction, and internal rotation) places the greatest strain on the anterolateral (AL) labrum (as does the lateral impingement position on the lateral labrum and does the posterior impingement position on the posterior labrum) (7,8). The “anterior to lateral” is the most common location for observation of acetabular labral tears due to cam and/or pincer morphology, whereas the “straight anterior “location is most commonly seen in patients with hip instability without dysplasia (9). In the setting of labral tears due to impingement, associated instability may also occur (10). Lastly, two distinct types of labral tears have been described; a partial to complete chondral-labral separation (Seldes 1) damage pattern, or a labral crush pattern with intra-substance injury (Seldes 2) (2).
In addition to instability, labral tears are a common cause of hip and/or groin pain. There are a number of different nerve fibers present in the labrum and at the chondrolabral junction that account for this finding (11). The neural anatomy of the labrum plays an important role in understanding a patient’s pain before and after surgery. In the normal labrum, free nerve endings (nociception) and nerve end corpuscle (Paccini, Golgi-Mazzoni, Ruffini, and Krause corpuscles; proprioception) are frequently identified from anterior to posterosuperior segments of the acetabulum (12-15). These fibers are primarily derived from the obturator nerve and the nerve to quadratus femoris. The nociceptive free nerve endings are predominantly found at the labral base, near the chondrolabral junction, decreasing further peripherally, most superficial on the labral surface (13). The continued presence of nociceptive fibers in a repaired labrum may permit residual post-operative pain due to the labrum. This is the principal rationalization for removal of native labral tissue and reconstruction with a neural graft (16). While removal of these nociceptive fibers is an advantage of labral reconstruction, the removal of proprioceptive mechanoreceptors may permit premature excessive early graft stress, increased failure risk, and should be considered during rehabilitation (16,17).
In addition to the importance of neural supply to a torn or healing labrum, vascular supply is equally essential. The labral blood supply is derived from the radial branches of the periacetabular periosteal ring, which come from the superior and inferior gluteal arteries (18). These vessels travel on the iliac periosteum, penetrate the capsule near the capsular insertion above the acetabular rim, continue on a loose connective tissue layer on the capsular side of the labrum, and terminate at the free edge of the labrum. There is no vascular contribution to the labrum from the capsule, synovium, or osseous acetabular rim. Macroscopic and histologic assessments have revealed stable repair healing and retention of the normal triangular shape without residual detachment (19). Additionally, neovascularization has been observed near the sutures supporting the repair. Further, although cam and pincer morphology associated with FAI syndrome and labral tears has been traditionally thought to be a purely “wear-and-tear” mechanical phenomenon, it has been significantly associated with inflammation and neovascularization as well (20-22). Labral biopsy specimens, obtained during hip arthroscopy or open osteoplasty for FAI syndrome and labral tear, have shown significantly greater macrophage (via CD68, CD206, IL-13), T-cells (CD3), mast cells, and vascular endothelium [CD34, vascular endothelial growth factor (VEGF)] than osteoarthritis labra (20). In addition, messenger ribonucleic acid (mRNA) expression of chemokines (IL-8, CXCL1, CXCL3, CXCL6, CCL3, CCL3L1), matrix-degrading [matrix metalloproteinase (MMP)-13 and ADAMTS-4], and structural matrix [COL2A1 (collagen, type II, alpha) and ACAN (aggrecan)] genes was higher in FAI syndrome hips than normal controls or hips with osteoarthritis (21).
Based on this anatomy of the labrum, surgical management has the potential to greatly affect its function. In the laboratory, labral tear (12 o’clock and 3 o’clock; 35 mm length), partial resection, and complete resection significantly decrease intra-articular fluid pressurization and maximal distraction force versus the intact labrum, respectively (3). Further, due to the role of the labrum in central compartment fluid pressurization and suction seal retention, a loss of labral function via focal and complete labrectomy significantly increases articular cartilage friction due to fluid exudation (23). Although labral repair using a pierced labral base refixation technique provides significantly greater increase in pressurization and maximum negative pressure generation versus a looped circumferential suture (3,24), no difference in clinical outcomes has been observed (25). Labral reconstruction with iliotibial band graft has been shown to increase pressurization similar to that of the intact labral state (3). However, labral reconstruction can improve distraction force (vs. partial labral resection), but not back to that of the intact labral state (24).
Patient evaluation
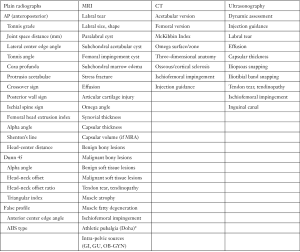
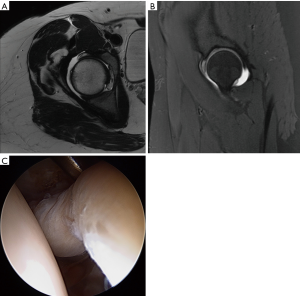
A thorough evaluation of the patient’s chief complaint and history of present illness should be combined with a comprehensive and systematic physical examination. Importantly, then and only then, can imaging [plain radiographs, magnetic resonance imaging (MRI), computed tomography (CT), or ultrasonography] be interpreted and a diagnosis rendered. This triad of patient symptoms, clinical physical examination signs, and imaging can be used to make a diagnosis of FAI syndrome per the Warwick Agreement (26). Symptoms are typically motion- and/or position-related hip and/or groin pain (can also be anterior thigh, lateral hip, buttock, and/or back). This is frequently described as a “C” sign or “between the fingers” sign. Clinical signs typically involve a combination of impingement maneuvers and range of motion assessment (Table 1). Loss of flexion, internal rotation, and the sum of internal and external rotation are frequently observed. The presence of cam morphology significantly decreases flexion, while a relative loss of femoral version significantly decreases internal rotation (27). Femoral version assessment is best assessed with a combination of gait analysis, foot-progression, and seated, supine, and prone internal and external rotation measurement. This is frequently combined with advanced imaging version measurement. Imaging assessment includes a combination of two-dimensional planar (plain radiographs, MRI, CT) and three-dimensional imaging (MRI, CT) (Figure 1). Clinicians must be cognizant of the high prevalence of asymptomatic imaging abnormalities in the general population and in specific athletic groups (29). The prevalence of labral tear has been reported to be approximately 62% (95% CI: 47% to 75%) in symptomatic individuals and 54% (95% CI: 41% to 66%) in asymptomatic individuals (30). Thus, clinicians must “treat the patient, not the X-ray”. Nonetheless, in individuals with FAI Syndrome, MRI (with or without arthrogram) is not a perfect imaging modality for detection of labral injury (sensitivity ranges from 50% to 90%) (Figure 2) (31,32). Higher magnet strength (greater than 1.5-Tesla), addition of radial series, oblique sagittal and oblique axial series, and arthrography increase the sensitivity of labral tear detection. Although intra-articular local anesthetic injections may be used as a diagnostic modality, their utility in prediction of post-operative labral surgery outcome after a positive injection response is limited (33-35). However, a negative response to injection is a strong predictor of a negative response to arthroscopic hip preservation labral surgery (36,37).

Full table
Indications and contra-indications
The indications for labral surgery include an adequate trial (minimum of 6 weeks to 3 months) and failure of non-surgical treatment. Further, the patient should be dissatisfied with the condition of their hip, meaning symptoms are unacceptable. Currently, there is no role for prophylactic hip preservation surgery in asymptomatic individuals with abnormal hip morphology and/or labral tear (38). Non-surgical measures may include education (activity modification), physical therapy, limited rest, non-opioid oral non-steroid anti-inflammatory medications and/or acetaminophen, and a variety of therapeutic injections (e.g., corticosteroid, platelet-rich plasma) (39). Physical therapy should emphasize posterior pelvic tilt to minimize dynamic impingement via gluteus maximus, rectus abdominis, and transversus abdominis activation; abductor control, optimized abductor/adductor strength ratio; optimized quadriceps/hamstring strength ratio; core and pelvic floor control. Improved sagittal balance with the spinopelvic alignment (with knowledge of pelvic incidence) should be stressed. Nonetheless, there is no evidence that has shown healing of labral injury or alteration of osseous morphology with any non-surgical measure. The majority (>90%) of labral tears are believed to be secondary to an osseous reason—cam, pincer, subspine, dysplastic, abnormal femoral version, abnormal neck-shaft angle morphologies (40-43). Even in the event of failure of physical therapy to sufficiently and satisfactorily relieve symptoms, the strength and/or motion gains achieved likely make post-operative therapy that much easier or more efficient due to the progression in therapeutic exercise learning curve.
Contraindications to labral surgery, as part of a comprehensive hip preservation procedure, are primarily contraindications to hip preservation surgery in general, rather than specifically to labral repair itself. Most labra, even those in advanced arthritic hips, can technically be “repaired”. However, the outcomes of arthroscopic hip preservation surgery, including labral repair, are inferior in patients with advanced arthrosis (Tonnis grade 2 or 3; joint space distance on weight-bearing anteroposterior pelvic radiograph less than 2.0 to 2.5 mm) (44,45). Thus, arthritis is a relative contraindication to labral repair. Arthroplasty is the more appropriate surgical treatment in advanced hip arthrosis. Dysplasia (defined via multiple imaging modalities and parameters: lateral center edge angle less than 18–20 degrees, anterior center edge angle less than 18–20 degrees, Tonnis angle greater than 15 degrees, femoral head extrusion index greater than 25%) is a relative contraindication to isolated “labral repair”. A broken Shenton’s line or excessive femoral head lateralization/subluxation are absolute contraindications to isolated labral repair, unless performed in conjunction (simultaneous or staged) periacetabular osteotomy. Patients with asymptomatic labral tears are contraindicated (absolute) for labral repair.
Author’s preferred technique for labral surgery
Patient positioning
The patient is positioned on a traction table (Advanced Supine Hip Positioning System with two Universal Hip Distractors and two Active Heel Traction Boots; Smith & Nephew, Andover, MA, USA). General anesthesia and muscle paralysis assists in force reduction to obtain sufficient distraction (greater than 10 mm) for atraumatic hip joint entry with a 70-degree arthroscope. Postless, gravity-assisted options for traction are available, but do require Trendelenburg positioning, a friction-based pad to prevent patient slippage, and adjustment in hand position during surgery, with its own associated learning curve (46). Since the most common complication of hip arthroscopy is iatrogenic chondrolabral injury (during portal placement), proper portal placement begins with meticulous patient positioning. Per Dr. Thomas Byrd, “Proper positioning gives you a reasonable chance to do well, while poor positioning guarantees failure” (personal communication). The risk of iatrogenic injury can be minimized, or even eliminated, with certain technical pearls (47-49). The hip arthroscopy learning curve, quantified via reduction in complication, reoperation, or total hip arthroplasty conversion rates, can be as little as 20 or as many as 519 independent hip arthroscopy surgeries (50-52).
Examination under anesthesia
A range of motion examination under anesthesia is performed, with attention paid to both hips, assessing hip flexion, internal and external rotation at 90 degrees of hip flexion, and abduction. A fluoroscopic examination can also be performed to better localize impingement pre- and post-correction of abnormal morphology. In addition to cam and/or pincer impingement, extra-articular sources of impingement, including ischiofemoral and trochanteric-pelvic impingement, can also be observed. External rotation recoil, dial, and axial distraction (with fluoroscopy) can assist with instability diagnoses. The presence of a vacuum sign can indicate a loss of the suction seal, primarily secondary to labral tear or deficiency (24).
Portal placement and capsulotomy
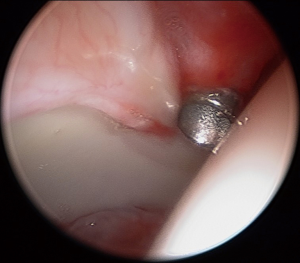
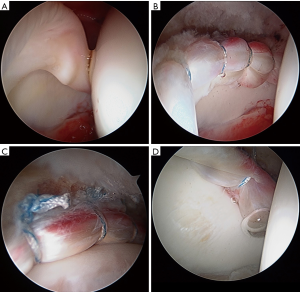
The AL portal is the first portal created, with fluoroscopic guidance and a 17-gauge spinal needle, to enter the joint at approximately the 12:30 position on the clockface. A 4.5-mm cannula is placed, using Seldinger technique, and a 70-degree arthroscope introduced into the joint. The author prefers dry arthroscopy until at least part of the interportal capsulotomy is created, to avoid a “red-out” or “pink-out” (a mixture of blood and fluid), which can obscure visualization. The anterior triangle needs to be visualized in order to accurately place a modified mid-anterior portal (MMAP) at the 2:30 to 3:00 position. If synovial fluid and/or tissue obscures visualization, then the scope should be removed, cannula retained, and the lens lightly wiped and re-inserted. Alternatively, a syringe of air may be injected into the joint to clear the field of view, or the syringe may be used to aspirate an effusion for improved visualization. Inability to obtain sufficient distraction and a large hypertrophic labrum (Figure 3A-C) make visualization of the anterior triangle and MMAP creation more challenging. Interportal capsulotomy is created using a 4.0-mm Beaver Blade (Smith & Nephew, Andover, MA, USA). The capsulotomy should be at least 7 to 8 mm away from the labrum, in order to preserve as much proximal capsular tissue as possible for closure at case conclusion—the key to repairing the capsule is preparing the capsule. The length of the capsulotomy needs to be big enough to adequately visualize the pathology in need of treatment, but no larger (on average, capsulotomy length is 2.0 cm length). A capsular suspension technique helps to lift the capsule (without iatrogenic capsulectomy for visualization) and visualize the acetabular rim and subspine region (53). Central compartment diagnostic arthroscopy then ensues once the interportal capsular incision is completed.
Labral repair
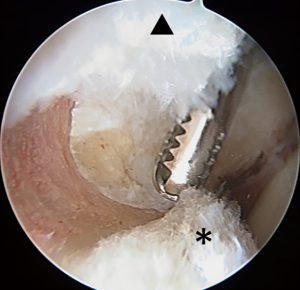
Acetabuloplasty rim trim is performed to treat pincer impingement (focal loss of cranial acetabular anteversion, retroversion, global pincer impingement) (Figure 4). If no pincer treatment is performed, then a minimal (less than 1 mm) rim decortication may be performed to smooth the rim for easier labral anchor placement, in addition to improved osseous vascular response to labral repair/refixation healing. No short-term clinical outcome difference has been observed with labral detachment and repair versus labral repair in situ without detachment (54). Labral repair can be performed using two or three portals. The author prefers a three-portal technique, with the addition of a spinal needle-localized distal anterolateral accessory (DALA) portal, placed approximately 4 to 5 cm distal to the AL portal. Suture anchor insertion needs to be performed without articular cartilage penetration (subchondral bone violation) or psoas tunnel perforation. The rates of articular surface penetration (4.0% mid-anterior vs. 5.0% DALA; P>0.999) or psoas tunnel perforation (4.2% mid-anterior vs. 11.6% DALA; P>0.25), in a cadaveric model, have not been shown to be significantly different (55). For anchor placement between 11 o’clock and 4 o’clock, the author utilizes the DALA portal. Anterior to the 4 o’clock position, a trans-capsular approach via MMAP is utilized. Posterior to 11 o’clock, a fourth portal [posterolateral (PL)] is created and used to perform posterior labral repair (less common) or reconstruction (more common). The author prefers an all-suture anchor construct for labral repair (Q-fix, Smith & Nephew, Andover, MA, USA). This particular anchor has demonstrated a 1.6% incidence of intra-operative anchor pullout over 434 cases (18 months) with 4.6 anchors placed per case (range, 1 to 8; total 2,007 anchors used) (56). This anchor has an active spherical deployment to approximately 3.5 to 4.0 mm diameter (utilizes a 1.8-mm drill that penetrates 22 mm deep), rather than a “pull-back” design. Typically, three to six anchors are used per case, spaced at least 7 mm apart (Figure 5). Most anchors are drilled and placed on the back side of the labrum (capsular non-articular side). Depending on the exact location of the clockface where the anchor is to be placed and the exact angle of the straight drill and anchor deployment mechanism, most anchors are placed approximately 2.0 to 2.5 mm off the chondro-osseous junction (acetabular rim). As the all-suture anchor actively expands to a 4.0 mm diameter (2.0 mm radius) and the drill/anchor diverge from the subchondral plate, a safe distance to avoid articular surface disruption is a minimum of 2.0 mm (57). Alternatively, an articular-sided approach may also be used where the bone is thinnest and subchondral bone violation (articular cartilage damage) or psoas fossa penetration is possible and at risk (Figure 6). Additionally, if an angle of anchor insertion or rim thickness does not permit anchor placement with a straight guide system, curved systems are available to place anchors. A curved system has been shown to be significantly more effective in increasing the angle of insertion of suture anchors and increasing the distance of the suture anchor tip to the articular cartilage at the 1 o’clock position on the clockface (58). The author prefers and usually (>99%) uses a straight system, but does have a curved system available if needed (Q-fix CURVED, Smith & Nephew, Andover, MA, USA).
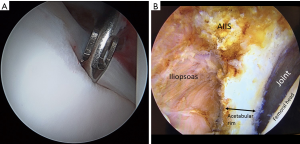
Once the anchor is placed, the suture configuration must be selected. The two primary available constructs are a circumferential looped repair (the suture wraps completely around the labrum) and a labral base refixation pierced repair (the suture goes through the labral substance. If the labrum is small (less than 4–5 mm) or of poor quality, then a circumferential looped repair may be a better choice. If the labrum is hypertrophic (greater than 10–12 mm), then a circumferential looped repair may also be a better option. If the labral tissue is of sufficient quality and is sized “not too big or too small”, then a labral base refixation pierced repair may be a better choice. Opponents of the looped repair espouse that the labral base refixation pierced technique does not evert the labrum and better restores the suction seal as it does not “spot-weld” the labrum at each suture location (59). Opponents of the labral base refixation pierced technique claim that the suction seal is still restored with the looped technique and that the pierced technique may damage the labral substance and the suture may rip through or pull out (60). Although in the laboratory, a labral base refixation technique does restore the normal triangular shape of the labrum better and pressurizes the joint more than the looped repair, no difference in clinical outcome has been observed (3,25).
Labral reconstruction
In the revision setting of labral deficiency after a previous debridement or failed repair, an arthroscopic labral reconstruction may be indicated. In the primary setting with a calcified/ossified labrum, global overcoverage pincer impingement, or an irreparable labral tear, although controversial, a primary arthroscopic labral reconstruction may be performed. However, the author prefers a labral debridement if restoration of the arthroscopic suction seal is observed. If no suction seal is observed, then a labral reconstruction may be indicated. Two techniques of labral reconstruction may be performed: one, a segmental reconstruction of the damaged/missing segment of labrum; two, a complete front-to-back reconstruction. No biomechanical studies have compared these two techniques in the ability to restore femoral head stability in the acetabulum—pressurization or resistance to distraction. No biomechanical studies have compared all graft types—autograft versus allograft. The author prefers an allograft peroneus longus, approximately 5.5 mm in diameter. The reasons for this selection are the relative resistance to swelling once placed in the arthroscopic environment, the quick efficient preparation time (less than 1 minute) that does not require an assistant, its durability to handling in the joint, and its firm composition that reduces/prevents “spot-welding” of looped suture. In the revision setting with labral deficiency, the author prefers a complete reconstruction from front-to-back (17,61). Acetabular rim length, in males and females, is variable and ranges from 13 to 16 cm (62). Typical graft lengths range from 9 to 13.5 cm. Anchors are placed from anterior to posterior, placed all at the same time, then passed and tied sequentially (as opposed to placing and tying one at a time). Anchors are spaced approximately one cm apart. The anterior two anchors are typically placed through the MMAP with a transcapsular approach rather than through the interportal capsulotomy. This avoids excessive fluid extravasation into the iliopsoas fossa and retains iliofemoral ligament integrity better than a more extended interportal cut. From approximately 3 o’clock to 11 o’clock, anchors are placed through the DALA portal. Anchors posterior to 11 o’clock are placed through a PL portal.
At the conclusion of labral management, traction is discontinued and the peripheral compartment is entered. A “T” capsulotomy is preferred, as it best offers visualization of the femoral head neck junction (63). Comprehensive cam correction is performed and completion is verified with an arthroscopic dynamic examination. Fluoroscopic dynamic examination may also be performed. However, this is associated with excessive radiation exposure, may be unnecessary, and is not significantly better than an arthroscopic dynamic examination (64). The T capsulotomy is closed side-to-side with high-strength non-absorbable #2 suture. Typically, this is either three or four sutures. The interportal capsulotomy is usually closed next (>98% of the time), with high-strength non-absorbable non-kevlar tape suture. If the patient is at risk for post-operative microinstability, then plication with greater bites of suture and an inferior capsular shift performed. Depending on capsulotomy size (usually 2 cm), two to four tapes may be used.
Post-operative management
A hinged hip brace is applied in the operating room, with 90 degrees flexion and 0 degree abduction as ends of permitted motion. The brace is recommended for 4 weeks. Derotational boots are applied and used for 2 weeks following surgery at night time. These prevent excessive external rotation while the hip is extended. Crutch-assisted partial (~20 pounds) weight-bearing is recommended for 4 weeks. At 4 weeks, an attempt of weight-bearing as tolerated is made. If no limp is observed or perceived, crutches are discontinued. Two crutches are continued until normal gait is achieved. There is no one crutch transition between two and zero crutches. Continuous passive motion (CPM) machine is recommended for 2 weeks following surgery. Compression-cryotherapy is recommended for 3 weeks following surgery. Formal physical therapy is recommended to commence on post-operative day #1. Gentle passive motion, circumduction exercises, limited rotation, and minimized iliopsoas activation are recommended.
Conclusions
The acetabular labrum serves an important biomechanical function in the hip. In patients with FAI syndrome, a variety of labral injuries are frequently observed. These may be the source of significant motion- and position-related hip and/or groin pain. However, there is a high prevalence of asymptomatic labral tear in the general and athletic population. Non-surgical treatment for symptomatic individuals includes education, activity modification, physical therapy, non-opioid oral medications, and injections. In the event of non-surgical treatment failure in non-arthritic and non-dysplastic individuals, surgical treatment includes labral repair and correction of cam, pincer, and subspine morphologies, and capsular management. Labral reconstruction is usually a revision procedure. However, in select primary cases (ossified labrum, global overcoverage pincer, irreparable tear), a reconstruction (or debridement) may be performed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Olufemi R. Ayeni and Ryan P. Coughlin) for the series “Future Perspectives in Hip Preservation and Arthroscopy” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.06.06). The series “Future Perspectives in Hip Preservation and Arthroscopy” was commissioned by the editorial office without any funding or sponsorship. JDH reports other from AAOS, other from AOSSM, personal fees from Arthroscopy: The Journal of Arthroscopic and Related Surgery, other from AANA, grants from Depuy Synthes, A Johnson & Johnson Company, other from ISAKOS, personal fees from SLACK, Inc., grants and personal fees from Smith & Nephew, personal fees from Xodus Medical, personal fees from Ossur, outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Draovitch P, Edelstein J, Kelly BT. The layer concept: utilization in determining the pain generators, pathology and how structure determines treatment. Curr Rev Musculoskelet Med 2012;5:1-8. [Crossref] [PubMed]
- Seldes RM, Tan V, Hunt J, et al. Anatomy, histologic features, and vascularity of the adult acetabular labrum. Clin Orthop Relat Res 2001;232-40. [Crossref] [PubMed]
- Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal--Part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc 2014;22:722-9. [Crossref] [PubMed]
- Dwyer MK, Jones HL, Hogan MG, et al. The acetabular labrum regulates fluid circulation of the hip joint during functional activities. Am J Sports Med 2014;42:812-9. [Crossref] [PubMed]
- Dwyer MK, Jones HL, Field RE, et al. Femoroacetabular impingement negates the acetabular labral seal during pivoting maneuvers but not gait. Clin Orthop Relat Res 2015;473:602-7. [Crossref] [PubMed]
- Crawford MJ, Dy CJ, Alexander JW, et al. The 2007 Frank Stinchfield Award. The biomechanics of the hip labrum and the stability of the hip. Clin Orthop Relat Res 2007;16-22. [PubMed]
- Ollivier M, Le Corroller T, Parratte S, et al. Mechanical strains passing through the acetabular labrum modify its shape during hip motion: an anatomical study. Knee Surg Sports Traumatol Arthrosc 2017;25:1967-74. [Crossref] [PubMed]
- Safran MR, Giordano G, Lindsey DP, et al. Strains across the acetabular labrum during hip motion: a cadaveric model. Am J Sports Med 2011;39:92s-102s. [Crossref] [PubMed]
- Shibata KR, Matsuda S, Safran MR. Is there a distinct pattern to the acetabular labrum and articular cartilage damage in the non-dysplastic hip with instability? Knee Surg Sports Traumatol Arthrosc 2017;25:84-93. [Crossref] [PubMed]
- Harris JD, Gerrie BJ, Lintner DM, et al. Microinstability of the Hip and the Splits Radiograph. Orthopedics 2016;39:e169-75. [Crossref] [PubMed]
- Kim YT, Azuma H. The nerve endings of the acetabular labrum. Clin Orthop Relat Res 1995;176-81. [PubMed]
- Haversath M, Hanke J, Landgraeber S, et al. The distribution of nociceptive innervation in the painful hip: a histological investigation. Bone Joint J 2013;95-b:770-6.
- Alzaharani A, Bali K, Gudena R, et al. The innervation of the human acetabular labrum and hip joint: an anatomic study. BMC Musculoskelet Disord 2014;15:41. [Crossref] [PubMed]
- Gerhardt M, Johnson K, Atkinson R, et al. Characterisation and classification of the neural anatomy in the human hip joint. Hip Int 2012;22:75-81. [Crossref] [PubMed]
- Kapetanakis S, Gkantsinikoudis N, Dermon A, et al. Normal microscopic architecture of acetabular labrum of hip joint: a qualitative original study with clinical aspects. Muscles Ligaments Tendons J 2017;7:279-85. [Crossref] [PubMed]
- White BJ, Stapleford AB, Hawkes TK, et al. Allograft Use in Arthroscopic Labral Reconstruction of the Hip With Front-to-Back Fixation Technique: Minimum 2-Year Follow-up. Arthroscopy 2016;32:26-32. [Crossref] [PubMed]
- White BJ, Herzog MM. Arthroscopic Labral Reconstruction of the Hip Using Iliotibial Band Allograft and Front-to-Back Fixation Technique. Arthrosc Tech 2016;5:e89-97. [Crossref] [PubMed]
- Kalhor M, Horowitz K, Beck M, et al. Vascular supply to the acetabular labrum. J Bone Joint Surg Am 2010;92:2570-5. [Crossref] [PubMed]
- Audenaert EA, Dhollander AA, Forsyth RG, et al. Histologic assessment of acetabular labrum healing. Arthroscopy 2012;28:1784-9. [Crossref] [PubMed]
- Elias-Jones CJ, Farrow L, Reilly JH, et al. Inflammation and Neovascularization in Hip Impingement: Not Just Wear and Tear. Am J Sports Med 2015;43:1875-81. [Crossref] [PubMed]
- Hashimoto S, Rai MF, Gill CS, et al. Molecular characterization of articular cartilage from young adults with femoroacetabular impingement. J Bone Joint Surg Am 2013;95:1457-64. [Crossref] [PubMed]
- Chinzei N, Hashimoto S, Fujishiro T, et al. Inflammation and Degeneration in Cartilage Samples from Patients with Femoroacetabular Impingement. J Bone Joint Surg Am 2016;98:135-41. [Crossref] [PubMed]
- Song Y, Ito H, Kourtis L, et al. Articular cartilage friction increases in hip joints after the removal of acetabular labrum. J Biomech 2012;45:524-30. [Crossref] [PubMed]
- Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal--Part II: The effect of an acetabular labral tear, repair, resection, and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc 2014;22:730-6. [Crossref] [PubMed]
- Sawyer GA, Briggs KK, Dornan GJ, et al. Clinical Outcomes After Arthroscopic Hip Labral Repair Using Looped Versus Pierced Suture Techniques. Am J Sports Med 2015;43:1683-8. [Crossref] [PubMed]
- Griffin DR, Dickenson EJ, O’Donnell J, et al. The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med 2016;50:1169-76. [Crossref] [PubMed]
- Kraeutler MJ, Chadayammuri V, Garabekyan T, et al. Femoral Version Abnormalities Significantly Outweigh Effect of Cam Impingement on Hip Internal Rotation. J Bone Joint Surg Am 2018;100:205-10. [Crossref] [PubMed]
- Weir A, Brukner P, Delahunt E, et al. Doha agreement meeting on terminology and definitions in groin pain in athletes. Br J Sports Med 2015;49:768-74. [Crossref] [PubMed]
- Frank JM, Harris JD, Erickson BJ, et al. Prevalence of Femoroacetabular Impingement Imaging Findings in Asymptomatic Volunteers: A Systematic Review. Arthroscopy 2015;31:1199-204. [Crossref] [PubMed]
- Heerey JJ, Kemp JL, Mosler AB, et al. What is the prevalence of imaging-defined intra-articular hip pathologies in people with and without pain? A systematic review and meta-analysis. Br J Sports Med 2018;52:581-93. [Crossref] [PubMed]
- Chopra A, Grainger AJ, Dube B, et al. Comparative reliability and diagnostic performance of conventional 3T magnetic resonance imaging and 1.5T magnetic resonance arthrography for the evaluation of internal derangement of the hip. Eur Radiol 2018;28:963-71. [Crossref] [PubMed]
- Rajeev A, Tuinebreijer W, Mohamed A, et al. The validity and accuracy of MRI arthrogram in the assessment of painful articular disorders of the hip. Eur J Orthop Surg Traumatol 2018;28:71-7. [Crossref] [PubMed]
- Krych AJ, Sousa PL, King AH, et al. Intra-articular Diagnostic Injection Exhibits Poor Predictive Value for Outcome After Hip Arthroscopy. Arthroscopy 2016;32:1592-600. [Crossref] [PubMed]
- Kivlan BR, Martin RL, Sekiya JK. Response to diagnostic injection in patients with femoroacetabular impingement, labral tears, chondral lesions, and extra-articular pathology. Arthroscopy 2011;27:619-27. [Crossref] [PubMed]
- Ladd LM, Keene JS, Del Rio AM, et al. Correlation Between Hip Arthroscopy Outcomes and Preoperative Anesthetic Hip Joint Injections, MR Arthrogram Imaging Findings, and Patient Demographic Characteristics. AJR Am J Roentgenol 2016;207:1062-9. [Crossref] [PubMed]
- Ayeni OR, Farrokhyar F, Crouch S, et al. Pre-operative intra-articular hip injection as a predictor of short-term outcome following arthroscopic management of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc 2014;22:801-5. [Crossref] [PubMed]
- Lynch TS, Steinhaus ME, Popkin CA, et al. Outcomes After Diagnostic Hip Injection. Arthroscopy 2016;32:1702-11. [Crossref] [PubMed]
- Collins JA, Ward JP, Youm T. Is prophylactic surgery for femoroacetabular impingement indicated? A systematic review. Am J Sports Med 2014;42:3009-15. [Crossref] [PubMed]
- Wall PD, Dickenson EJ, Robinson D, et al. Personalised Hip Therapy: development of a non-operative protocol to treat femoroacetabular impingement syndrome in the FASHIoN randomised controlled trial. Br J Sports Med 2016;50:1217-23. [Crossref] [PubMed]
- Dolan MM, Heyworth BE, Bedi A, et al. CT reveals a high incidence of osseous abnormalities in hips with labral tears. Clin Orthop Relat Res 2011;469:831-8. [Crossref] [PubMed]
- Guevara CJ, Pietrobon R, Carothers JT, et al. Comprehensive morphologic evaluation of the hip in patients with symptomatic labral tear. Clin Orthop Relat Res 2006;277-85. [Crossref] [PubMed]
- Peelle MW, Della Rocca GJ, Maloney WJ, et al. Acetabular and femoral radiographic abnormalities associated with labral tears. Clin Orthop Relat Res 2005;327-33. [Crossref] [PubMed]
- Wenger DE, Kendell KR, Miner MR, et al. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res 2004;145-50. [Crossref] [PubMed]
- Domb BG, Gui C, Lodhia P. How much arthritis is too much for hip arthroscopy: a systematic review. Arthroscopy 2015;31:520-9. [Crossref] [PubMed]
- Philippon MJ, Briggs KK, Carlisle JC, et al. Joint space predicts THA after hip arthroscopy in patients 50 years and older. Clin Orthop Relat Res 2013;471:2492-6. [Crossref] [PubMed]
- Mei-Dan O, Kraeutler MJ, Garabekyan T, et al. Hip Distraction Without a Perineal Post: A Prospective Study of 1000 Hip Arthroscopy Cases. Am J Sports Med 2018;46:632-41. [Crossref] [PubMed]
- Domb B, Hanypsiak B, Botser I. Labral penetration rate in a consecutive series of 300 hip arthroscopies. Am J Sports Med 2012;40:864-9. [Crossref] [PubMed]
- Domb BG, Botser IB. Iatrogenic labral puncture of the hip is avoidable. Arthroscopy 2012;28:305-7; author reply 307-8. [Crossref] [PubMed]
- Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy 2013;29:589-95. [Crossref] [PubMed]
- Mehta N, Chamberlin P, Marx RG, et al. Defining the Learning Curve for Hip Arthroscopy: A Threshold Analysis of the Volume-Outcomes Relationship. Am J Sports Med 2018;46:1284-1293. [Crossref] [PubMed]
- Weber AE, Harris JD, Nho SJ. Complications in Hip Arthroscopy: A Systematic Review and Strategies for Prevention. Sports Med Arthrosc 2015;23:187-93. [Crossref] [PubMed]
- Coughlin RP, Ayeni OR. Educating the future arthroscopic hip surgeon. Ann Joint 2018;3:13. [Crossref]
- Spiker AM, Camp CL, Barlow BT, et al. Capsular Preservation Using Suture Suspension Technique in Hip Arthroscopy for Femoroacetabular Impingement. Arthrosc Tech 2016;5:e883-e887. [Crossref] [PubMed]
- Redmond JM, El Bitar YF, Gupta A, et al. Arthroscopic acetabuloplasty and labral refixation without labral detachment. Am J Sports Med 2015;43:105-12. [Crossref] [PubMed]
- Degen RM, Poultsides L, Mayer SW, et al. Safety of Hip Anchor Insertion From the Midanterior and Distal Anterolateral Portals With a Straight Drill Guide: A Cadaveric Study. Am J Sports Med 2017;45:627-35. [Crossref] [PubMed]
- Byrd JWT, Jones KS, Loring CL, et al. Acetabular All-Suture Anchor for Labral Repair: Incidence of Intraoperative Failure due to Pullout. Arthroscopy 2018;34:1213-6. [Crossref] [PubMed]
- Hernandez JD, McGrath BE. Safe angle for suture anchor insertion during acetabular labral repair. Arthroscopy 2008;24:1390-4. [Crossref] [PubMed]
- Nho SJ, Freedman RL, Federer AE, et al. Computed tomographic analysis of curved and straight guides for placement of suture anchors for acetabular labral refixation. Arthroscopy 2013;29:1623-7. [Crossref] [PubMed]
- Fry R, Domb B. Labral base refixation in the hip: rationale and technique for an anatomic approach to labral repair. Arthroscopy 2010;26:S81-9. [Crossref] [PubMed]
- Lertwanich P, Ejnisman L, Philippon MJ. Comments on “Labral base refixation in the hip: rationale and technique for an anatomic approach to labral repair”. Arthroscopy 2011;27:303-4; author reply 304. [Crossref] [PubMed]
- White BJ, Herzog MM. Labral Reconstruction: When to Perform and How. Front Surg 2015;2:27. [Crossref] [PubMed]
- Karns MR, Patel SH, Kolaczko J, et al. Acetabular rim length: an anatomical study to determine reasonable graft sizes for labral reconstruction. J Hip Preserv Surg 2016;4:106-12. [PubMed]
- Nho SJ, Weber A, Kuhns B, et al. A T-Capsulotomy provides increased hip joint visualization compared to an extended interportal capsulotomy: implications for improved capsular management. J Hip Preserv Surg 2016;3:hnw030.002.
- Smith KM, Duplantier NL, Crump KH, et al. Fluoroscopy Learning Curve in Hip Arthroscopy-A Single Surgeon's Experience. Arthroscopy 2017;33:1804-9. [Crossref] [PubMed]
Cite this article as: Harris JD. Strategies in managing the labrum. Ann Joint 2018;3:57.


